Alloy Formation, Methods of Production, And Its Various Applications

''Alloy Formation, Methods of Production, And Its Various Applications''

Practically every material we would ever need is sneaking someplace in the planet underneath our feet. From the gold we wear as adornments to the oil that powers our autos, Earth's storage facility of astounding materials can supply every need. Compound components are the essential building obstructs from which every one of the materials inside Earth is made. There are 90 or so normally happening components, and the dominant part of them are metals. However, valuable metals are, they're in some cases not as much as ideal for the jobs we require them to do. Take irons, for instance. It's incredibly solid. However it can be very fragile, and it additionally rusts effortlessly in the moist air. Or, then again shouldn't something be said about aluminum. It's light at the same time, in its unadulterated frame, it's too delicate and frail to be of much utilize. That is the reason the majority of the "metals" we utilize are not metals at everything except rather alloys: metals joined with different substances to make them more grounded, harder, lighter, or better in some other way. Alloys are wherever around us—from the fillings in our teeth and the alloy wheels on our autos to the space satellites zooming over our heads. How about we investigate what they are and why they're so valuable!

Alloys are usually classified as substitutional or interstitial alloys, depending on the atomic arrangement that forms the alloy.
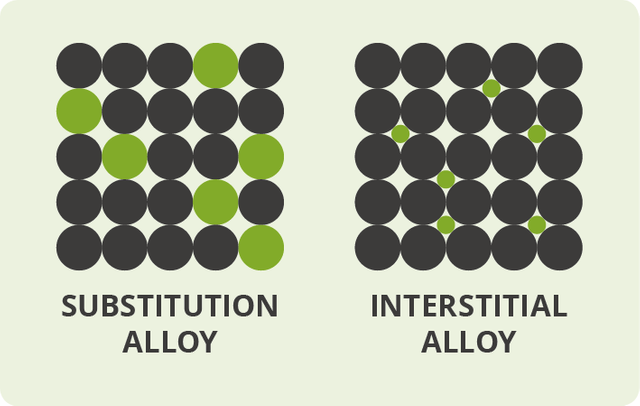
Substitution alloys
On the off chance that the molecules of the alloying agent replace particles of the primary metal, we get what's called a substitution alloy. An alloy like this will form only if the particles of the base metal and those of the alloying agent are of generally comparative size. In most substitution composites, the constituent components are very close to each other in the periodic table. Brass, for instance, is a substitution alloy based on copper in which atoms of zinc replace 10– 35% of the particles that would ordinarily be of copper. Brass acts as an alloy since copper and zinc are near each other in the periodic table and have atoms of generally comparable size.
Interstitial alloys
Alloys can likewise produce if the alloying agent has atoms that are especially smaller than those of the original metal. The agent atoms slip in the middle of the fundamental metal atoms (in the gaps or "interstices"), giving what's called an interstitial alloy. Steel is a case of an interstitial alloy in which a moderately modest number of carbon molecules slip in the gaps between the gigantic atoms in a crystalline lattice of iron.
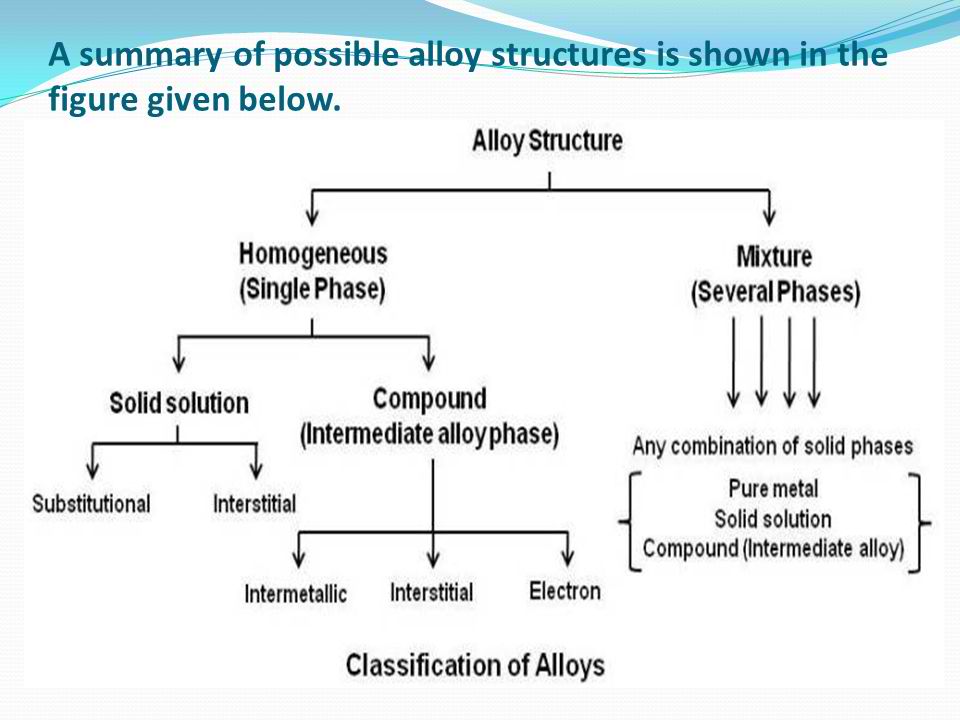
At the point when an alloy comprises of two segments, it is known as a binary alloy. An alloy with three parts is a ternary alloy, and an alloy with four segments is a quaternary alloy. The subsequent metallic substance has properties that are essentially unique with those of its parts. Altering the arrangement by 1% can change the property of one unit of an alloy framework. Based on its development, the alloy can be named:
Homogeneous alloy
Mixture or heterogeneous alloy
In a homogeneous alloy, the blend comprises of just a single phase, and a mixture alloy is a mix of a few phases.
There are a few alloys of different metals, for example, an alloy of Potassium, Aluminum, Iron, Nickel, Copper, Silver, Tin, Gold, Cobalt Mercury, Lead, Bismuth, Gallium, Zirconium, and uncommon earth. Because of the presence or nonappearance of Iron, combinations can be arranged into:
Ferrous alloy: Contain Iron as a noteworthy component. A couple of cases of ferrous alloys are Cobalt, Silver, Gallium, Bismuth, Gold, Zirconium, and
Stainless Steel,
Non-ferrous compounds: Do not contain Iron as a noteworthy part. For instance, Brass, Aluminum, Bronze, Tin, Nickel, Copper, Titanium, and Magnesium are some regular non-ferrous alloys.
Pure metals have a couple of essential physical and metallic properties, for example, breaking point, melting point, flexibility, particular gravity, thickness, high pliability, and warm and electrical conductivity. These properties can be changed and improved by alloying it with some other metal or nonmetal, as per the need.
Alloys are made to enhance the hardness of metal, modify color, lower the melting point, enhance tensile strength, and provide better castability. Alloying a metal increases the inertness of the metal, which, in turn, increases corrosion resistance.
There are four customarily utilized methods for the production of alloys: the powder metallurgy, the fusion method, reduction method, and the electro-deposition method.
Powder Metallurgy
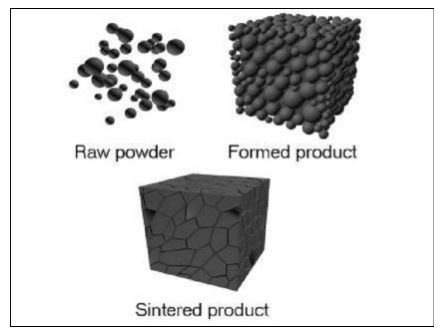
Powder metallurgy might be characterized as the specialty of creating fine metal powders and afterward making articles from blending alloys or elemental powders and compacting the blend in a die, the resultant shapes are then warmed or "sintered" in a controlled environment heater to bond the particles metallurgically. The high exactness framing ability of powder metallurgy produces parts with near net shape, unpredictable highlights and great dimensional accuracy pieces are frequently completed without the need for machining.
The Fusion Method

This method utilizes alloying components in a settled extent and fuses them together in a refractory melting pot or brick-lined crucible. The part metal with a higher dissolving point is softened first and afterward, the other segment with a lower dissolving point is added to the dissolve. Both metal parts are blended well and permitted to liquefy further. The molten mass is secured by powdered Carbon to keep away from the oxidation of the molten alloy component since they are incredibly receptive to the encompassing air oxygen. The subsequent molten mass is permitted to cool to room temperature.
The Reduction Method
A metal may exist as mixes. The reduction is a chemical procedure in which a compound of one part can be isolated from another part, to get an unadulterated (pure) metal. This technique is performed in an electric furnace.
The Electro-Deposition Method
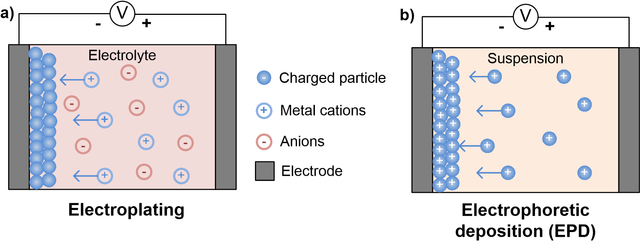
This technique includes synchronous deposition of various part metals from the electrolytic solutions containing their salts solutions blend by passing direct electricity.
Alloy has been utilized as a part of ventures for quite a while. Hardly any broadly utilized applications are:
Stainless Steel is utilized as a part of the wire and lace frames for applications, for example, staple, catheter, belt, cable, weld, screening, suture wire, and metalizing.
An alloy of Silver and Gold are utilized as a part of the preparation of jewelry. White Gold, which is an alloy of Palladium, Gold, Silver, and Nickel is utilized as a cheap alternative to Platinum. A wide selection of alloy is utilized as a part of welding applications by various enterprises.
Some alloy work as materials corrosion resistant and are utilized as a part of moisture rich-conditions. High-temperature alloys have been utilized for petrochemical applications and aerospace. Likewise, they have been utilized for welding wire, where hoisted temperatures and brutal conditions are routinely experienced. These alloys have been utilized as a part of uses where corrosion protection and high quality must be kept up at lifted temperatures.

Moreover, magnetic alloys are utilized for magnetic cores and dry reed switches. Quality control measures incorporate magnetic testing to keep up reliably elevated requirements of consistency and execution. Alloys are additionally used to create inside and outer leads. Iron-Chromium-Aluminum, Nickel-Chromium, and Nickel-Chromium-Iron alloys have been utilized for high-temperature warming components. Some alloys are utilized as protection components to control or measure electric current. Applications have included wire-wound resistors, rheostats, potentiometers, and shunts.
Thermocouple alloys have discovered an extensive variety of utilization in temperature detecting and control. Alloys are likewise utilized as radio and electronic devices, automotive applications, thermostat metals, precision devices in airship controls, and telecommunications.
References:
https://en.m.wikipedia.org/wiki/Alloy
https://byjus.com/chemistry/transition-metals-alloy-formation/
http://www.explainthatstuff.com/alloys.html
http://www.globalspec.com/reference/46874/203279/html-head-chapter-1-structure-properties-and-applications-of-various-alloys
https://www.epma.com/what-is-powder-metallurgy
Recent Articles:
Congratulations @lymierikxz, this post is the second most rewarded post (based on pending payouts) in the last 12 hours written by a Newbie account holder (accounts that hold between 0.01 and 0.1 Mega Vests). The total number of posts by newbie account holders during this period was 2450 and the total pending payments to posts in this category was $1112.85. To see the full list of highest paid posts across all accounts categories, click here.
If you do not wish to receive these messages in future, please reply stop to this comment.
Thanks, @bitgeek :)
Resteemed by @resteembot! Good Luck!
Curious?
The @resteembot's introduction post
The @reblogger's introduction post
Get more from @resteembot with the #resteembotsentme initiative
Check out the great posts I already resteemed.
Thanks @resteembot :)
Whatever @resteembot resteems, I resteem too!
I am a new, simple to use and cheap resteeming bot
I will automatically resteem posts resteemed by @resteembot until 2017-12-26 00:00:01 +00:00
If you want to read more about me, read my introduction post.