Battery Science Part II: The Guts of a Battery
Welcome to the second issue of Battery Science! If you are new here I recommend that you start with my first post in the series (Steemit.com) so you have a bit of background before jumping into the nitty gritty! Through this post, I hope to convey a bit about the components of a battery by way of an Anatomy Analogy. Here we go!
1. The Brain – Electrical Controller (PLC)
Batteries are dumb. Really dumb. So dumb, in fact, that while I have stated in the title of this section that they have a brain, they really don’t. Batteries are brainless zombies that do whatever the electrical controller or programmable logic controller tell them to do. Otherwise, batteries would have no control over themselves! [1];[2]
 Image Credit: Pixabay.com
Image Credit: Pixabay.com
2. The Arms and Legs – Electrodes
The below diagram does a good job detailing the direction of ion and electron flow at each of the electrodes, as well as the layout of a typical cell.
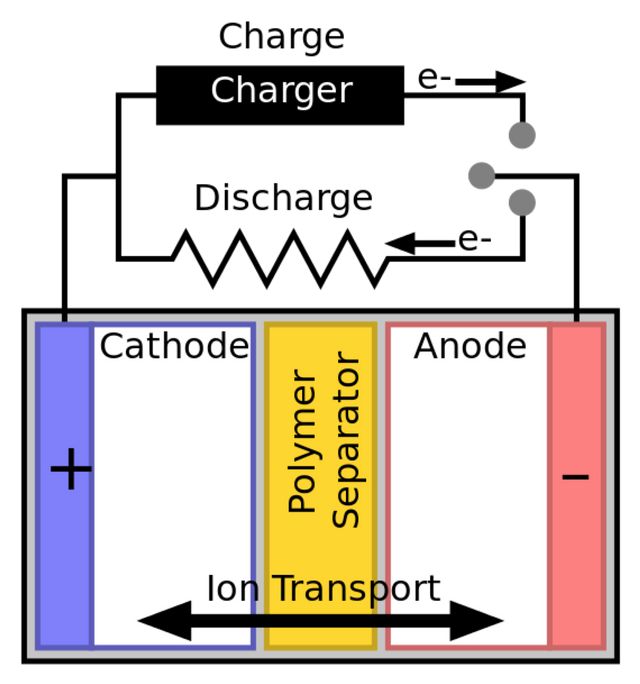
Image Credit: Wikipedia.org Author: Tkarcher CC BY-SA 3.0
Every battery is made up of cells, which are essentially the repeat unit of electrochemical storage. Each cell is made up of two electrodes that are typically aligned to be parallel to one another. Each cell has one cathode and one anode which are the positive and negative electrodes, respectively. The electrodes do the heavy lifting of the cell, mainly storing 'electricity' in the form of charge. Each electrode material is coated onto current collectors (see below) and usually consists of some combination of polymer binder to hold it together in combination with metals. [2];[3]
3. The Central Nervous System – Current Collectors & Wiring
Image Credit: Pexels.com
Current collectors are typically copper or aluminum foils much like you would see in a household kitchen. Current collectors and wiring are used to transport electrons quickly to drive the chemical reaction (when charging) or to be used upon the opposite reaction (discharging). Current collectors have electrode materials coated on them in order to store the electricity and maintain good contact to electrical sources or loads. [2];[3]
4. The Skin – Package

Image Credit: Pexels.com
Like humans, batteries come in all sorts of shapes and sizes. The packaging or skin, if you will, depends primarily on the chemistry and the application. For example you could have a cylindrical lithium ion battery, a prismatic (read: boxy) lead acid battery, or a nickel metal hydride button/coin cell. The packaging itself uses different materials with the most common ones being plastics, aluminum, or steel. [2];[3]
5. The Blood – Electrolyte

Image Credit: Pexels.com
The life of the battery is ensured by the electrolyte. Typically, an aqueous or organic salt mixture, electrolytes allow for fast movement of ions (charged particles) between electrodes. These ions (usually having a positive charge) are met upon arrival to the opposing electrode by a negatively charged electron. This concept of positive ions and negative electrons meeting one another at each electrode (depending on whether it is charging or discharging) is called electroneutrality and is always maintained. [2];[3]
6. The Ligaments – Separator

Image Credit: Pexels.com
Separators are typically very thin polymer and/or ceramic membranes that act to keep everything in place, much like a ligament would. The separator keeps the two electrodes from touching each other while still allowing ions to travel between the electrodes. This enables the storage of electricity at one electrode at a time without the meltdown that would occur if the two electrodes made contact. [2];[3]
Fin.
If you have questions, feel free to comment and I will answer to the best of my ability. If you enjoyed this post I would appreciate any upvotes, follows, or resteems.
If you didn’t like this post, tell me how I can improve! :)
HUGE thanks to @scienceangel for her help and @steemstem for being awesome!
Sources:
[1] Thermal-Electrical Modeling and Adaptive Control of Battery Charge/Discharge Systems by Renato Oliveira de Magalhaes and Marcelo de Oliveira E Souza
[2] The handbook of lithium-ion battery pack design : chemistry, components, types and terminology by John Warner
[3] Lithium Batteries by Christian Julien, Alain Mauger, Ashok Vijh, and Karim Zaghib
Well batteries are quite wonderful... But could be dangerous if not taken good care of.
Agreed! Especially lithium batteries!
You can embed your link by using this markdown
[LINK](URL)There is no space between the square and conventional brackets. Eg
[Google](https://www.google.com)will come off as Google.Thanks for the tip!
Congratulations @eloos! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last announcement from @steemitboard!
Congratulations @eloos! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!