Chemistry Express: How Atoms Begin to Associate

When studying chemistry, people often start by memorizing the names of chemical elements and compounds or learning how to balance chemical equations. However, all this feels very abstract and nonsensical if one cannot grasp the following general concept: How do atoms begin to associate with each other in the first place? What causes them to react with certain atoms and “ignore” others? This is one of the most important concepts to comprehend in order for things to make some sense. I will therefore try to simplify it as much as I can.
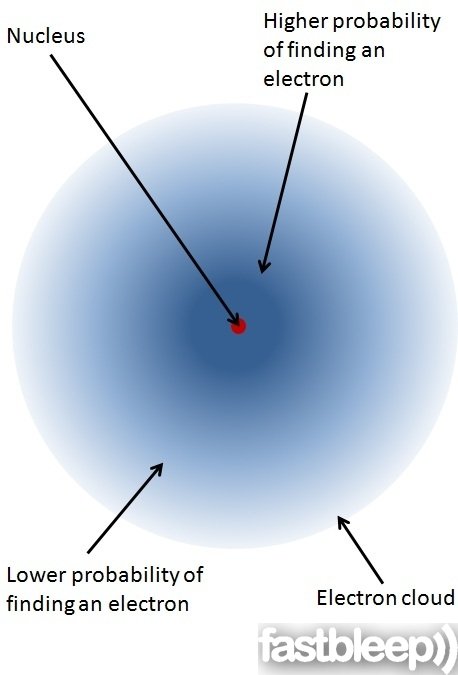
In the center of the atom there is the positively charged nucleus, made of protons and neutrons. Negatively charged electrons buzz around the nucleus in random paths. These paths are called orbitals.
An electron’s orbital around an atom is not like a planet’s orbit around the sun. Celestial bodies move in an elliptical path well-defined by gravity, while electrons just move around randomly. We can never know for sure where exactly an electron is at a particular moment! However, we can calculate where it will most likely be most of the time.
Electrons distribute themselves around the atom in what we call energy shells, that is, energy levels: Electrons with lower energy usually move close to the nucleus. Others with higher energy are mostly further away. Their position depends on two things: how much energy they have at that moment, and how much “space” there is left in a particular shell. Electrons don’t enjoy crowded places because, as they all carry negative charges, they repel each other!
A great example to illustrate the concept of energy shells can be found on this video. Let’s say that we have a comet moving around the sun. The comet circles the sun in a close orbit. If we gave it an extra boost of energy with the help of a rocket booster, it would gain kinetic energy. That would make it accelerate a bit faster and run a larger orbit around the sun. If we added a second rocket booster, the orbit around the sun would become even larger!
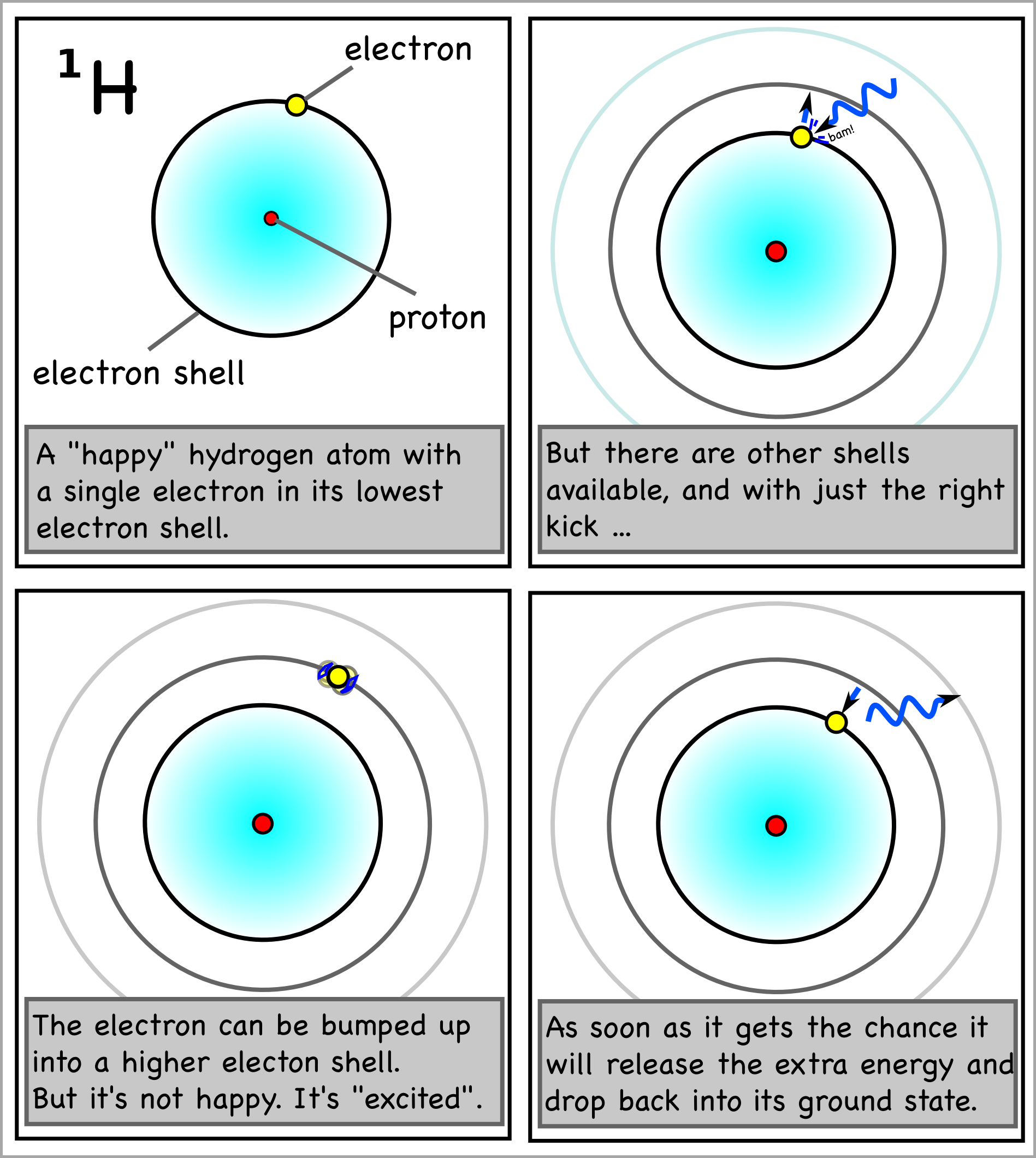
Similar to our example, when electrons get hit by electromagnetic waves such as light photons and receive extra energy, they enter a higher energy state and get pushed further away from the nucleus. Obviously, as explained before, a comet’s orbit is not the same thing as an electron’s orbital. This analogy is only used for illustration purposes.
Electrons don’t really "like" being in a higher energy state. Their favorite place is towards the center and near the nucleus, as they are attracted by its positive charge. If the electron gets the chance, it will jump back to a lowest energy state and emit the photon with its extra energy back. (And that’s why some things glow!)
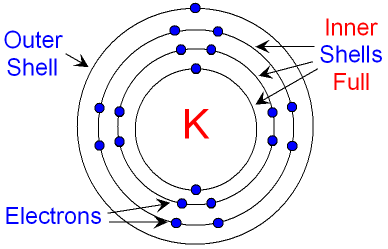
But what if it's already too crowded? Only two electrons can fit in the lowest energy shell! From there onward, only eight electrons are allowed to fill each consecutive shell. Once filled with its eight electrons, each shell is now stable and "sealed". Any remaining electrons will have to move into an even higher energy shell.
If an energy shell does not have it’s eight “seats” filled, it is unstable. The electrons of this outermost shell are called valence electrons. Valence electrons are the electrons which have the highest energy and are the furthest away from the nucleus. Usually, highest energy automatically means they are also the furthest out, with some exceptions we will see on a next lesson.
The valence electrons of an atom are the most likely to react with other atoms and form bonds. They can be swept away, or shared with other atoms. These are the electrons that “count” in chemical reactions, the ones we draw when we draw the formation of chemical bonds!
Here is the basic key to understanding chemical reactions: All atoms want to be stable, so they always try to have eight electrons in their outermost shell, either by “donating”, “robbing”, or sharing electrons with other atoms. For example, if an atom has just one valence electron, it will most likely choose to “give it away” to an atom with seven valence electrons, just so they can both remain with a complete outer shell each.
...And this is how chemical reactions start to happen and chemical bonds to form!

Thanks for another informative mini-class :)
Steem on !
Thank you! :)
Thanks again for the wonderful explanation. You nailed it again. Upvoted :)
Btw, nowadays, teachers start throwing concept of spdf right there. My younger bro was telling me that he learnt about protons and electrons in his physical science class, and their teacher said that this 'orbit' concept is not correct, they should learn the 'orbital' from the very beginning. I was highly surprised. Okay, maybe his teacher wants to tell the students everything, but students should actually get the basic idea before jumping into those stuff.
How we can exite an electron and make it jump from a lower exitation state to a higher exitation state, and their tendency to loose energy and come down... these concepts needs to be developed. You explained it well. :)
Thank you, @nirmal :) I agree that for kids, it can be really difficult to grasp the idea of a probability distribution without even knowing the basics of classical mechanics yet! I know that in most science classes kids get taught about the Bohr model first, which is a good basic approach. However, the concept of energy shells and valence electrons is the necessary basis for all the other things that come next in chemistry in my opinion. Electronegativity, periodic groups, bond types... even oxidation numbers etc. These are the "boring theory" for some, but it actually saves a lot of memorization and a lot of "why?!"s. Personally, when I started studying chemistry, electron configurations was the first thing I learned. It took me ages and a lot of effort, but after that, everything seemed to make sense :)
Hmm. You are correct. :)
You should join steemstem channel in chatroom!
I just have. Thank you for the invitation :)
Great work!! Love this as a different way to introduce the basic of chemistry without forcing the first 20 elements down an unsuspecting kids throat. Your post has been selected to be featured on the Science-Trail, keep up the good work!
Join us on the Science Discord