Chemistry Express: Electronegativity

Electronegativity is a chemical property describing the tendency of an atom to attract electrons and bind with them. In basic words, it is basically measuring how strongly a particular atom attracts electrons.
Conceptually, electronegativity is the opposite of ionization energy. The term electronegative takes its name from the fact that it attracts electrons, which are negatively charged particles. An electronegative atom will make electrons spend more time with them than around other atoms in a bond.
Take for example the bond of water. A molecule of water is made by one oxygen atom with six valence electrons, and two hydrogen atoms with one valence electron each. All three will share their electrons, in order for the oxygen to reach the eight electrons needed to fill up its outer shell, and for the hydrogens to reach two electrons each in order to fill up their own shells (remember that the first energy shell is the exception and only needs two electrons to be completed!).
However, even if everyone is sharing their electrons, oxygen does not have the same electronegativity as hydrogen. This means that the oxygen attracts more the shared electrons of the molecule and makes them spend more time around it than around the hydrogens. This creates a partially negative charge around the oxygen atom of the molecule, and partially positive charges around the hydrogens, creating what we call a polar molecule. Water is a polar molecule, which gives it many of the properties it has!
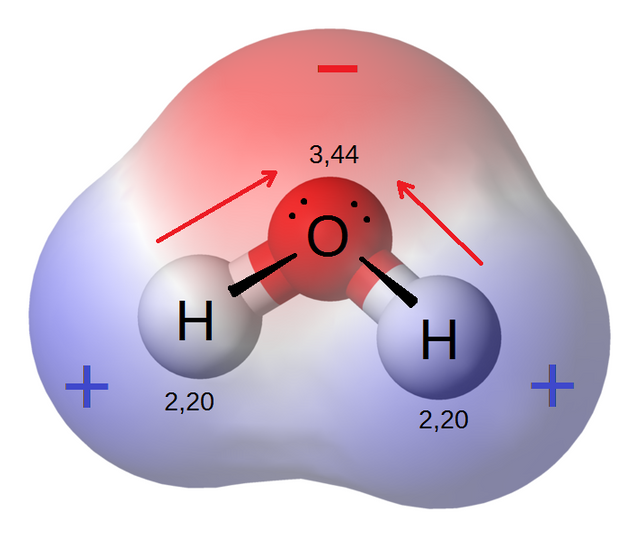
image credit
Why are some atoms more electronegative than others?
Electronegativity is determined partly by the amount of protons an atom has inside the nucleus, or the nuclear charge. This is because the more protons there are inside the nucleus, the stronger the positive charge; in turn, this will attract a stronger negative charge.
Electronegativity is also partly determined by the number of electrons an atom has, because if there are many electron shells around the atom, the valence electrons will be relatively far away from the nucleus and not able to experience its pull that much. This is even more enhanced by the fact that the negative charge from all the electrons of the atoms repulse both the valence electrons and the electrons of a second atom since they have the same negative charge! This phenomenon is called electron shielding.
Finally, electronegativity depends on the amount of valence electrons an atom has. This is because if the valence shell of an atom is less than half full, less energy is needed to lose these few electrons and reach the next complete shell, rather than try to fill the last shell up. On the other hand, if the shell is more than half full, it is easier to fill it up by attracting a few more electrons.
Electronegativity trends
We can observe these trends on the periodic table of elements. Firstly, across a period of elements, electronegativity increases from left to right, as the amount of valence electrons increases. Secondly, electronegativity decreases from top to bottom down a group, as the distance and the electron shielding take effect.
We see that as we move from left to right on the periodic table, atoms become more electronegative. They like to attract electrons more, in order to complete their outer shells. Atoms on the left are not very electronegative, since they’d rather just give up their few electrons and remain with the next complete shell of electrons. This is the case for most metals.
Noble gases is the only group that has no electronegativity, since they already have eight electrons in their outer shells and don’t want to attract or lose any!
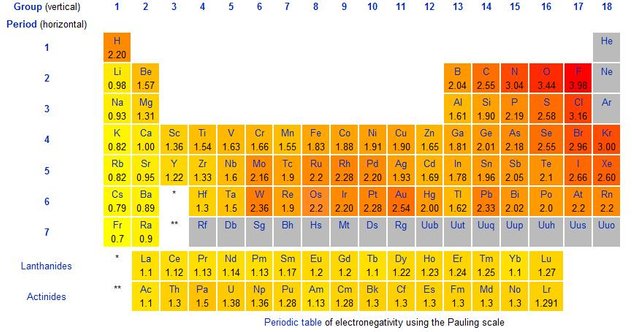
image credit
Size matters
It is important to note here that an atom with a lot of valence electrons defeats to some degree the shielding effect, and the atomic radius that defines the size of an atom decreases across a period. This is because as the nucleus is increasing in protons across a period, valence electrons of the same energy level are increasing as well, and the nuclear charge pulls them closer to the nucleus.
The opposite happens as you move down a group and the number of electron shells increases. Each shell is further away from the nucleus, and the atomic radius increases. So, in general terms, electronegativity tends to increase as the atomic size decreases, and vice versa.

his story is good
This is not a good way to reply to posts. You have to read the post and write a related comment. Otherwise it's better not to write anything. This is spam and people will flag you.
sorry to learn ni still not gerti