This stuff will kill you

When I'm not being a sarcastic jerk on steemit, I like to read up on chemistry and physics. In particular I like the dangerous stuff and stories about when it went horribly horribly wrong. I'm going to share a few of the more interesting ones here with you today.
WARNING: IMAGES AND SUBJECT MATTER BELOW MAY BE DISTURBING TO SOME READERS
Chlorine trifluoride
I love this stuff, it's so stupidly dangerous. Chlorine Triflouride (ClF3) is either a colorless gas or greenish-yellow liquid depending on the circumstances and is said to smell "sweet, pungent, irritating, and suffocating" but I'll be damned if I know how anyone smelled it and lived to tell people what it was like. Because ClF3's big claim to fame is that it is INSANELY reactive. How reactive? Well, here's some test footage
There's a bit of a trend here: literally everything this stuff touches catches fire and explodes. Including glass, Teflon, sand, concrete, asbestos and WATER. And when it reacts with a substance it doesn't just burn and explode, it releases copious amounts of poisonous gas! If you get it on your flesh then not only will your flesh catch fire, but the reaction will produce Hydrochloric and Hydroflouric acid!
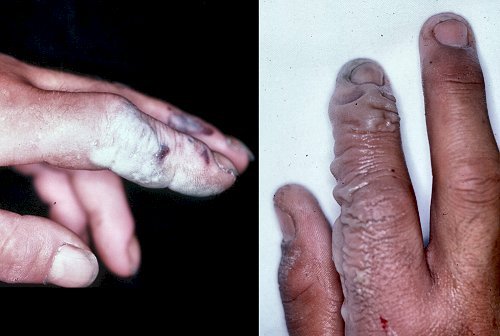
The result of Hydroflouric acid exposure.
The worst part about it is that ClF3 doesn't need oxygen to burn; it's actually a more effective oxidizer than oxygen itself. This means that conventional fire suppression methods either don't work or make things actively worse, literally adding fuel to the fire.
ClF3 was investigated as a chemical weapon in Nazi Germany and they actually began production on it under the name "Substance N", but the factory making it was captured by the Russians before it could ever be used. In America it was investigated during the space race as one of a number of stupidly reactive chemicals that could be used as oxidizers for fuel. In his book Ignition! An Informal History of Liquid Rocket Propellants John Clark describes it like this:
"It is, of course, extremely toxic, but that's the least of the problem. It is hypergolic (Ignites spontaneously) with every known fuel, and so rapidly hypergolic that no ignition delay has ever been measured. It is also hypergolic with such things as cloth, wood, and test engineers, not to mention asbestos, sand, and water — with which it reacts explosively. It can be kept in some of the ordinary structural metals — steel, copper, aluminum, etc. — because of the formation of a thin film of insoluble metal fluoride which protects the bulk of the metal, just as the invisible coat of oxide on aluminum keeps it from burning up in the atmosphere. If, however, this coat is melted or scrubbed off, and has no chance to reform, the operator is confronted with the problem of coping with a metal-fluorine fire. For dealing with this situation, I have always recommended a good pair of running shoes."
Unsurprisingly the ended up not using ClF3 in rockets, but it does have one modern use: it is used to clean the inside of chemical vapor deposition chambers in the semi-conductor industry. It has the advantage that, unlike other chemicals which need SUPER HEATED PLASMA to effectively clean the chamber, ClF3 can do it all on its own.
Amatoxin

The "Destroying Angel" mushroom, major source of amatoxin and current record holder for most metal name for a fungus.
Amatoxin is a complex and subtle poison, with side effects that most clearly mirror the side effects of radiation poisoning. Amatoxin interferes with one specific protein in the human body, namely RNA Polymerase II, an enzyme used during the process of DNA transcription. What this effectively means is that the toxin interferes with your body's ability to create new proteins by throwing a wrench into one of the early steps of the process. Without the ability to produce proteins, your cells quickly lose functionality and die. β-amanitin, one of several forms of Amatoxin, is so toxic that it can burn exposed skin rapid cell death.
The worst part about Amatoxin is that death from it, like radiation poisoning, tends to be a slow and unpleasant affair, and also one that there is no good cure for. The effects first show up in tissues like the lining of the intestine and the hepatocytes of the liver, cells that are involved in digestion and that have either a lower turnover time or a constant need to produce many different proteins. The lining of the intestines will sough off and die while the liver becomes grossly swollen by inflammatory factors released by immune cells trying to clean up the exploded remains of hepatocytes. Generally death comes from side effects related to liver damage, but the damage can be felt throughout the body. And there is no no cure for amatoxin, only supportive care and the hope that the patient will overcome it on their own. And any toxin with a treatment plan of "Hope and Pray" isn't one you want in your body.
Azidoazide Azide
Azidoazide Azide, besides sounding like the name of a Persian pop band, is a nitrogen compound with this as its chemical formula

Now, any of you with some chemistry knowledge would look at that and say "That doesn't exist. That can't exist." And you'd be partially right; because this is a compound that really seems to hate being around. And it makes sense; The thing is nothing but a giant mass of non-triple bonded nitrogens crammed onto two lonely carbons, treating valence shells like the porn industry treats orifices. It takes a massive amount of energy to form these tenuous bonds and all that energy is just waiting to spring back out when the bonds break, making the substance incredibly explosive. How explosive? Well, so explosive that the team that made it was literally unable to test the properties of the substance because it would explode every time they tried. When they tried to move it, it exploded. When they tried to touch it, it exploded. When they put it in direct light, when they dissolved it in solution, when they tried to look at the infrared spectrum of it, and even when they did absolutely nothing at all, it exploded.
There's not a lot that can be said about Azidoazide azide, because so little is known about it and we can do so little with it. Only a tiny amount was produced by Klapötke's team at the University of Munich, and chances are it will never be produced again as it has no useful purpose. It hold the dubious honor of being the most explosive material ever manufactured by man.
White Phosphorus

White phosphorus burns slightly and glows in the dark when exposed to oxygen
White phosphorus, also know as WP and Willie Pete, is fairly innocuous looking in a laboratory environment. It tends to look something like this

Almost like a block of bee's wax or maybe some kind of fancy cheese. If you hold it with a pair of tongs and look at it, you'll see that it smolders and smokes slightly on exposure to air. Interesting, but not exactly threatening. Light it on fire though, and all that changes.

WP bombardment
White Phosphorus is a potent weapon because it burns quite powerfully and cannot be easily extinguished or removed from the body. Water can be used to extinguish it so long as it is fully submerged, but as soon as it has any contact with oxygen it will reignite. WP borrows down into flesh as it burns, leaving characteristic "pot hole" wounds that don't heal well.
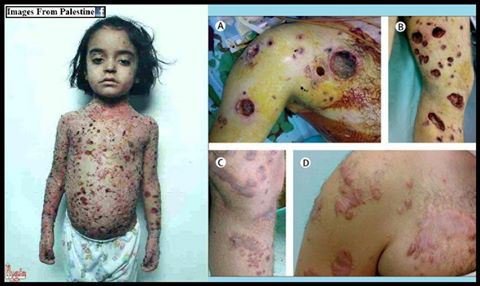
And whats worse is that the phosphorus is absorbed into the body through the wounds and this can cause damage to the liver, heart and kidneys. Many people with large scale WP burns die of multiple organ failure due to phosphorus poisoning rather than the actual tissue damage from the burns.
Although its use as an Incendiary weapon is theoretically banned by several treaties it continues to be employed by several military forces, including the US army, Israeli forces, and allegedly the Ukraine forces during the War in Donbass.