Matter
Hello steemians, let's swim onto science and let's explore our planet. Today's discussion is matter..... Almost everything on earth is matter.... Let's check through the concepts...
( )
)
Living matter has properties of respiration, growth, movement, metabolism (eating and excretion) and reproduction Growth in non-living matters only comes if there is an addition of the same or different matter.
CONCEPT OF MATTER Matter is anything that occupy space possesse mass of its own ,offers resistance to change of inertia and may by any of our sensory organs. Matter can exist in any of the three physical states which are solid, liquid, gas or plasma Matter takes its own shape when in solid form It takes the shape of the container when in liquid form (and flows when poured) and occupy all available space as gas or plasma KINETIC THEORY OF MATTER
The word Kinetic stands for motion Molecules is the smallest unit, consisting of a group of atom (one or more atom) Matter can exist in mixture or pure substance Matter in the pure form exists as an element such as hydrogen, Oxygen, Nitrogen etc. or compounds such as water, carbon (IV) oxide and ammonia. Matter as a mixture could be homogenous such as solution (e.g. salt in water) or heterogeneous mixture chocolate or soil. An element has only one type of atom e.g. hydrogen About 118 element exists in nature and are found naturally in one of the three physical states of matters e.g. Mercury, bromine__liquid, Sodium, copper__solid, Hydrogen__gas A compound has more than one type of atom bounded together chemically which can only be separated by a chemical process. All matter is capable of change from one physical state to another due to temperature change experienced by the matter or chemical reaction At times , matter change from solid to liquid and to gas but in few others it change from solid to gas without passing through liquid state Such substances are said to be SUBLIME and the process of change is SUBMLIMATION.
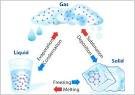
E.g. Iodine and ammonium chloride. The state of matter are distinguishable by the temperature and appearance of the matter Example is water in solid form as ICE, in liquid form as WATER and in gaseous state as STEAM Fusion is bonding together of two nuclei while fission is the division of nucleus into two or more nuclei Nuclear energy is energy released during a nuclear reaction.
CHAIN REACTION Occur when the process of one reaction leads to another with both source and product initiating the next reaction and resulting in an avalanche.
Still your humble steemians @dickiebash