Chemistry Basics
Welcome everyone to my new article in which i am going to explain the chemistry's basics, which i believe are the Atom, Periodic table of Elements, the Atomic number and the Mass number. I ll make it as simple as i can and try not to tire. So with no farther delay let us begin.

(Image Source: http://study.com)
The Atom
Our world is built out of atoms, from the smallest seed to the most complex structures. The types of atoms are many and varies. There are even some artificial man made atoms. Every atom is unique and has its own different mass, size and properties(that is in comparison to other atoms).
Atoms are making up the Elements. There are 118 different elements on the periodic table and 92 of them are naturally occurring on earth. The elements that do not occur naturally are artificially made in lab and can not occur in nature because they are too unstable for our planet's conditions.
(Image Source: https://nuclear-energy.net)
The Periodic Table of Elements
It is the creation of Dmitri Mendeleev, it displays the 118 known elements and organizes them by their properties as well as their atomic structure. The presentation of the elements is based by the increasing atomic number. For example Hydrogen has atomic number 1 and atomic mass 1.0079 and oganesson has atomic number 118 and atomic mass 294(most stable isotope and still not so stable).
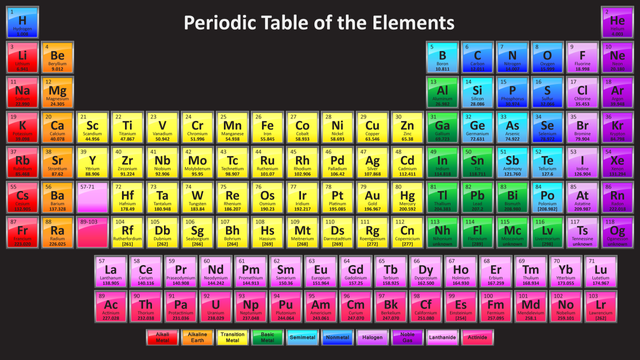
(Image Source: https://sciencenotes.org)
The Atomic Number
You should have noticed by looking at the periodic table that each element has a number above it. It is the atomic number of the atom. Which is the number of protons found in the nucleus of the atom. The proton number is also the same for the electrons of the atom in a natural occurred atom. The atomic number is symbolized by the letter Z.
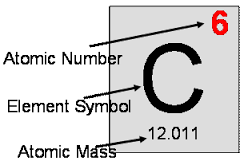
(Image Source: https://o.quizlet.com)
The Mass Number
Not to be confused with the atomic number. It is consisted by the number of Protons plus the number of the Neutrons found in the atom. It is placed right under the atomic symbol. It is symbolized by the letter A and so Z+N(Neutrons)=A.
Source Links:
https://www.thoughtco.com/how-many-elements-found-naturally-606636
https://www.nde-ed.org/EducationResources/HighSchool/Radiography/atomicmassnumber.htm
Thanks for reading feel free to follow, vote and Re-esteem if you find it useful.
You can find more articles of mine here: @diasdr For all personal thinking content, i am sorry if you find it in any way offensive, i didn't mean to offend anyone just to discuss/express my opinion.