How small are the atoms?
What's the size of an atom?
Have you ever wondered how big (or how small) are the atoms? Let's think.
An atom is very very small... as small that in the width of a hair, we can place 500.000 carbon atoms stacked over each other. So now have you got an idea?
How to understand how small is an atom
Let's imagine that an atom is as big as a marble. Now, let's think about the millions of atoms we have on our hand. If this were real, our hand would be as big as our planet is. Isn't this amazing? As Kurzgesagt said in his video, if our finger tip were the size of a room. This room will be filled with rice, and now every rice grain is a cell of our body. And now, a rice grain will be the size of the room. Again, we fill this room with rice, but this time we are going to fill the space between every rice grain with sand. Well, an atom would be as small as a sand grain is in that case.
What are atoms made of?
An atom is formed by three main elements: neutrons, protons and electrons. The protons and the neutrons form the core, and the electrons orbit around the core. Actually, the core is formed by other particles even smaller than the proton, electron or the neutron.
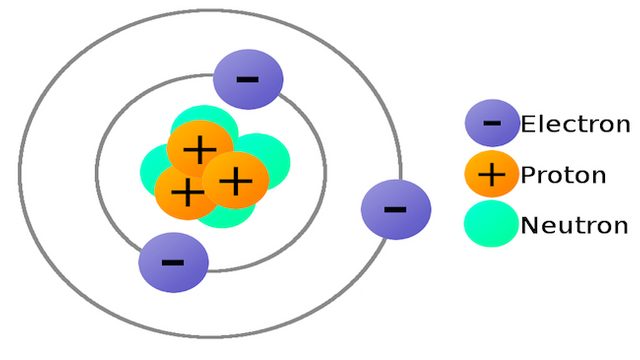
Sadly, the pics we find on the Internet are not the reality. These are simplifications so we can understand how atoms are. Actually, atoms are 99.9999% empty space, so the images that we usually see are unreal.
References
Images from Google Images
https://newsela.com/read/elem-sci-atoms
https://learn.sparkfun.com/tutorials/what-is-electricity/flowing-charges
Information:
Take a look at this amazing video if you want to learn more about atoms:
www.youtube.com/watch?v=_lNF3_30lUE