How Glue Sticks - The Chemistry
In the most basic sense, glue 'sticks things together'. Children come to terms with the idea of stickiness at a very young age, and most quickly come to associate stickiness with glue - in the form of Pritt sticks and PVA.
For such a simple concept, the science behind glue is surprisingly tricky.
In fact, the majority of scientists disagree on the true mechanism of stickiness.
Adhesives
Glues, or adhesives, have been put to work by humans for thousands of years. Most primitive glues were natural adhesives, relying on the innate stickiness and viscosity of a substance rather than any reactive adhesive effects.
Some traditional natural adhesives include tar, sap and beeswax.
For a while, glue derived from animal connective tissue was common - boiling down abattoir leftovers was a cheap and efficient method of producing gelatin and other adhesives. While this marked a transition away from natural adhesives and towards synthetic options, advancements in chemistry and the subsequent development of artificial adhesive production methods heralded the eventual collapse of the animal-based glue industry.
Nowadays, artificial adhesives, a more effective and cost-effective option, are prevalent. There is a variety of artificial adhesive types, but the majority fall into the category of reactive adhesives which depend on chemical reactions to cure or harden.
Stickiness
An effective adhesive must have two important properties. Cohesion is the process by which a material 'sticks' to itself; water is a cohesive substance, and cohesive forces are responsible for the surface tension that allows small insects to 'skate' on water and forms water droplets. Adhesive substances have the ability to stick to other substances. In water, these internal cohesive forces are stronger than adhesive forces, so water forms droplets rather than a film when in contact with a window.
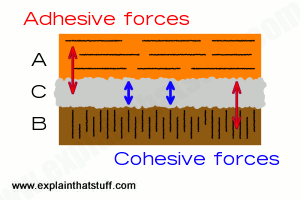
An effective adhesive must have acting forces at every boundary, some adhesive and some cohesive.
Cohesion
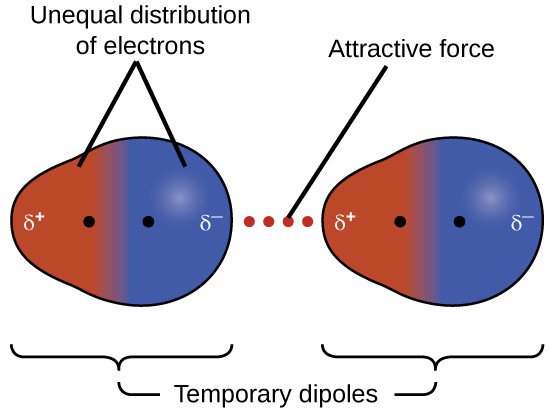
Cohesion is the result of attractive intermolecular forces at play within a substance. Three such forces responsible for attraction between molecules are Van der Waal's forces, permanent dipole-dipole forces and hydrogen bonding (the latter of which has been discussed in previous articles). Van der Waal's forces are the result of temporary close proximities between atoms of differing polarities belonging to different molecules - in essence, regions of varying electron density within a vibrating molecule will temporarily find themselves very close to adjacent areas of contrasting electron densities.
This phenomenon produces attractive forces between the disparate regions of every substance. However, these forces - also referred to as induced or temporary dipole interactions - are very weak and occur at distances so tiny that they any attraction is extremely transient.
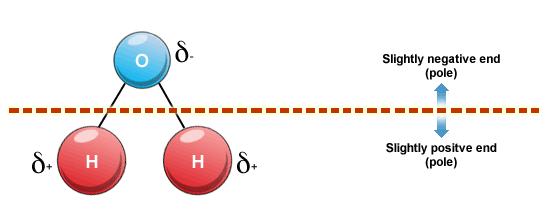
Permanent dipole-dipole forces occur only between polar molecules (molecules with permanently disparate regions of electron density, such as water) but by the same mechanism as Van der Waal's forces.
Asymmetry and electronegativity differences within a molecule are required for a molecule to be considered polar.
These attractive forces are stronger than Van der Waal's forces since they are permanent.
Hydrogen bonding occurs between hydrogen atoms and highly electronegative atoms (nitrogen, oxygen and fluorine) within an adjacent molecule, and is the strongest attractive force between molecules.
Again, the mechanism of attraction between the regions of differing electronegativity is the same.
Adhesion
Agreement on mechanisms of cohesion is pretty unanimous among scientists, and the chemistry on which they are based has been accepted theory for many decades.
Adhesion, however, is every bit as divisive as cohesion is uniting. There are two main schools of thought explaining adhesive chemistry.
Chemical Adhesion
Many scientists believe that the main mechanism of action in adhesion is a chemical one; the main processes behind chemical adhesion are adsorption and chemisorption.
Adsorption works best in low-viscosity ('runny') fluids. Such adhesives are able to cover the entirety of the substrate (the surface to which the glue is sticking) with a thin film, maximising the area of molecular contact. This allows the same intermolecular forces responsible for cohesion to provide adhesive forces between the glue and substrate.
While these forces are relatively weak, a compensatory increase in contact surface area greatly increases the attractive force between adhesive and substrate. These attractive forces are not strictly bonds but attractions.
Chemisorptive adhesives form real chemical bonds at the substrate-adhesive interface, generally due to a chemical reaction such as polymerisation (the joining of many mers or molecules into a larger molecule of repeated mer units).
These reactions are usually condition-dependent, which is why many glues do not work in the extreme hot or cold, or when the substrate is wet. These changes also alter the extent of intermolecular adsorptive (and cohesive) forces, further reducing the efficacy of an adhesive.
Mechanical Adhesion
Another theory is one of mechanical action, predicated on the idea that even smooth surfaces are, at least at the molecular level, rough.
Adhesive liquids seep into cracks and deformities - before hardening by whichever curing process is responsible for adhesive reaction - acting like grappling hooks and applying a mechanical force to keep the substrate and adhesive bound.
While this mechanism generally depends on chemical hardening (although non-hardened adhesive liquids may still provide enough intermolecular cohesion to mechanically 'hook' deformities), the prevalent forces at the interface in this model of adhesion are physical rather than electrostatic.
Diffusive forces, a sort of 'mingling' of molecular composition at the surface boundary, may also play a role, but evidence for this is less convincing than for mechanical adhesion.
A Cohesive Picture
It is almost universally accepted that the real science of adhesion is a mixture of these two schools of thought, chemical and mechanical, although the weighting of each theory remains a bone of contention among scientists unlucky enough to spend considerable amounts of time pondering the secrets of glue.
One Final Question
Now for a question that often crops up: 'why doesn't glue stick to the inside of the container?'
The answer lies in the fact that modern day glues rely on an additional curing process to harden and bind substances after adhesion.
To prevent adhesives drying in their tubes, a solvent is often added. This solvent prevents the chemical process responsible for curing but evaporates when released from the container.
A polar solvent also interferes with intermolecular forces responsible for adhesion, mitigating any attraction to the container wall substrate.
Some adhesives also harden by oxidation, and so must be stored in airtight containers.

If you enjoyed this article and would like more, follow the everyday science blog for your daily dose of science.
References:
www.explainthatstuff.com
www.chemistryexplained
www.wikipedia.org
James May's BBC Earth Lab featuring Vsauce
Interesting read! I'm surprised that even experts don't agree on how things stick to other things. Sometimes when I buy a tube of super glue, after using a little bit of it, the whole thing hardens as it dries up. So I'm not able to use animal of the super glue.