Homeostasis and Water Balance: How to survive a shipwreck and stay healthy under the principle of osmosis
Have you ever wondered how our body reacts when we eat an extremely salty meal? Or when drinking sea water in a hypothetical shipwreck? When ingesting an energizing drink like a gatorade without being really dehydrated? Here we explain what happens inside our body in such situations.
First recall some details and basic concepts, our body is composed of 60% water divided into 2 fundamental compartments, the intracellular 40% and the extracellular 20% the latter contains interstitial fluid and plasma. The rest of our body will be constituted by solutes such as salts, lipids and proteins.
Basically we are a dilution of substances in water

https://www.nlm.nih.gov/exhibition/historicalanatomies/valverde_home.html
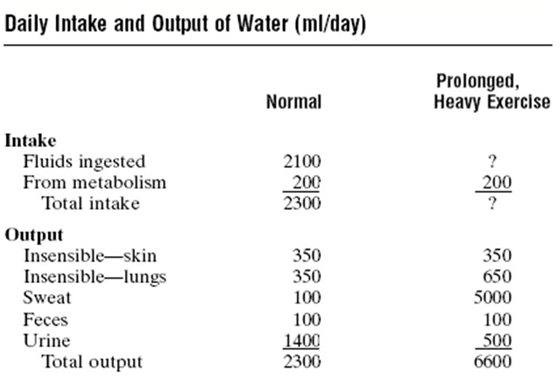
All at least once we have heard since childhood the famous phrase "* you must drink water so you do not get sick *" and yes, whoever mentioned it to you is right. Our body must maintain a water balance within itself, that is, the amount of liquid eliminated must be compensated by the amount of liquid ingested and thus there should not be any deficit. That is why we need to drink at least 2 liters of water per day, this figure can vary according to the climate and daily activities that are carried out, in addition 300 ml of water produced internally from the metabolism of the organic compounds of the cells. Similarly, daily the body will be responsible for eliminating the same amount of liquid either as losses called insensitive as breathing or sensitive losses through sweat, feces and urine. In such a way the balance between intake and output of water in the body is maintained.
http://slideplayer.com/slide/9086147/
And yes, it is very important to drink water!
Then already mentioned our body volumen, we need to add our solutes to complete the following equation:
C = M / V
Concentration = Mass / Volume
As we know, our body has a certain amount of substances that are kept in this sea that we can call internal medium (extracellular fluid) and even those same substances are inside our cells. Now, it is important that these elements are maintained in a certain balance and in adequate concentrations inside and outside the cells for the proper functioning of the organism. In a healthy person and under normal conditions, the human body maintains that dynamic balance within itself through what we call Homeostasis.
Therefore, these substances must be maintained in certain constant values, as for example in the extracellular liquid, the concentration of Sodium (Na +) is 135-145 milliOsmoles / L and in the intracellular of 14 milliOsmoles / L, the Potassium (K +) by on the contrary, its intracellular concentration is higher, 140 milliOsmoles / L and in the extracellular 4 milliOsmoles / L, and so it is with the other elements present in the organism.
| Solutes | Extracelular Fluid | Intracelular Fluid |
|---|---|---|
| Na+ | 135-145mOsm/L | 14mOsm/L |
| K+ | 4,2mOsm/L | 140mOsm/L |
| Cl⁻ | 108mOsm/L | 4mOsm/L |
| Glucose | 6mOsm/L | --------- |
| Urea | 4mOsm/L | 4 mOsm/L |
Now it is important to know that in our body, the total concentration of the substances both in the intra and extracellular medium should be 290 milliOsm / L, a very important number to consider as we will see later because if it is drastically modified this figure, the mechanisms of the cell to achieve balance can lead to its destruction.
Osmolarity: Concentration of the osmotically active particles contained in a solution expressed in milliosmoles per liter of solvent, understood as osmotically active particles those that do not pass the plasma membrane (at least not by simple diffusion) of the cell. There is only water movement (osmosis occurs). For example, glucose and urea are considered osmotically non-active particles because they pass freely into the cell interior
But how does the cell maintain a state of equilibrium?
Recall quickly the principle of osmosis which is the movement or flow of water between two compartments separated by a semipermeable membrane (impermeable to the solute). The direction of water movement is always higher to lower concentration and does not require energy.
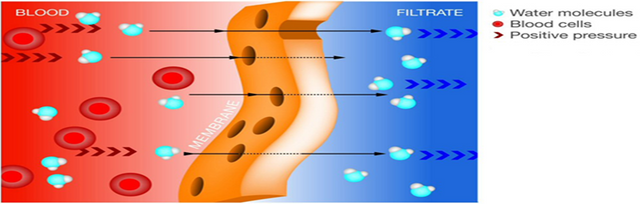
Our body will always seek balance and water will move according to its chemical or concentration gradient to establish it. On the other hand, when there is an disequilibrium in the electrolyte balance because it increases or decreases the extracellular osmolarity for different reasons, the cell can become dehydrated due to loss of water or, on the contrary, to swell due to excessive water intake.
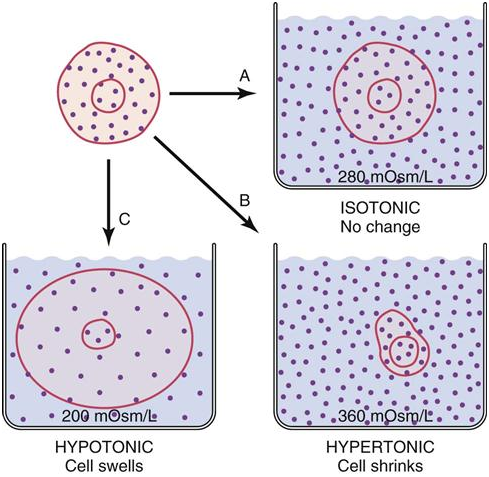
So it is important to understand how the substances we ingest in our body behave. For example, pure water, we can call it a hypoosmolar and isotonic substance since it will not produce changes in the concentrations of our corporal compartments. It will be a momentary increase in the volume of extracellular fluid that will be compensated by the flow of water inside the cell, maintaining the same concentrations.
Now if a hypertonic and hyperosmolar substance is ingested, that is to say highly loaded in solutes impermeable to the membrane, will be generated a higher concentration of solutes in the extracellular liquid and a lower concentration of water, which leads to the water coming out of the cell by concentration gradient causing it to become dehydrated. The opposite occurs if a hypoosmolar and hypotonic substance is ingested, since this will increase the concentration of solvent in the extracellular fluid, decreasing the concentration and producing the flow of water now inside the cell.
https://nursekey.com/fluid-electrolyte-and-acid-base-balance/
Understanding this, let's analyze our initial cases.
1- What happens when we eat an extremely salty meal: French fries

https://womanitely.com/i-ate-french-fries-week-what-happened/
At once we assume that they have a high amount of sodium chloride (NaCl), we can say that this food is hyperosmolar, with very high concentration, hypothetically 310miliOsm / L, then the potato chips enter our body establishing an increase in the level of ions sodium and chloride in the extracellular liquid increasing the osmolarity of the medium, that is, decreasing the concentration of water, in this situation our body will look for the way to establish the balance by diluting the concentration of solutes. Obviously the simplest option is to look for the water where it is nearest, the cell, therefore the water starts to come out and the cell becomes dehydrated, causing the sensation of thirst. Without much complication you should only drink water and thus set back normal concentrations in body compartments.
2- But what happens in a desperate situation, like being shipwrecked on the high seas?
With this unbearable desire to ingest some liquid and ironically you are surrounded by millions of liters of water, the sensation of thirst will gradually increase because the water outflows in your body exceed the income, the high temperature produces a greater sweating of up to 2 liters in a matter of hours and also adds the metabolic action of the body that still produces urine. In short, everything leads to dehydration.

https://www.safarisports.com/products/mr-wilson-volleyball
As we have seen previously, the option to drink seawater must be totally discarded. Well we know that the concentration of salts in seawater is grossly high for us, at the time of ingesting seawater our body will simply increase its osmolarity and the dehydration that your body previously already has, causing death. That is why the most sensible thing is to reduce both the amount of energy consumed and the temperature in your body to decrease sweating as much as possible, you can for example get wet with seawater and wait to be rescued.
What about the camels in the desert?
As we know in the desert, the situation of dehydration is not very different than in the high seas, the temperature is extremely high and it can even be worse, but what happens with those mammals that live in such conditions (?). The camel is an animal that has an incredible ability to walk miles at these temperatures to find the nearest oasis, the secret lies in its hump, which is made up of lipids that provides the water necessary to survive. These lipids are metabolized providing what is necessary to keep the camel hydrated and endure those marathon hours without liquid.

https://misanimales.com/diferencias-entre-camellos-y-dromedarios/
We are always hydrated!
3- Water vs Gatorade
I'm sure we've all had the craving to drink something very cold and refreshing and we should not necessarily be a castaway or wander in the desert. So we usually go to the nearest store and buy a very cold drink, perhaps as a Gatorade, excited by the taste of fruits and eager to quench our thirst. The only detail is that unfortunately we will end up thirstier than we arrived. These drinks are highly loaded in glucose and additional salts, therefore we will increase the osmolar concentrations in the extracellular liquid as in the previous cases and we will not quench the thirst, but what if we finished exercising and lost a lot of liquid? Well, the solution is to drink water. But why? Simply because our body usually already possesses the necessary salts and substances acquired in food and then reabsorbed in the kidney, eliminating those amounts that we do not need. Therefore, by ingesting any of these drinks we will be adding unnecessary solutes, making it even more difficult to establish the desired balance. However these electrolytic drinks are effective for high performance athletes such as a marathon runner, professional swimmers and boxers or a person who trains several times a day doing very high intensity exercises, since their electrolyte losses added to the physical requirement is greater than an ordinary person.

https://www.healthunit.com/drink-water-perform-better.com
That is why in this article we induce you to drink enough water during the day, 2 liters without fail and if you exercise remember to compensate for the lost liquid. Remember that it is not necessary to fall into the marketing of drinking some energy drink to improve your performance, adjust your diet, drink water and you will be healthy. That's all for now, we'll be waiting for you in our next article. Do not forget to vote and follow us. Until next time.
Postdata: English is not my native language, any inconvenience I apologize.
References:
Guyton and Hall. Treaty of Medical Physiology. 12th edition
https://www.healthunit.com/drink-water-perform-better.com
http://www.baxter.com.sg/patients_and_caregivers/therapies/renal/old_acute_kidney_treatment/continuous_renal_replacement_therapy.html