High-rate electrochemical energy storage Pseudo capacitive oxide materials
CLICK HERE Download Full paper from Google dive
Electrochemical energy storage technology is based on devices capable of exhibiting high energy density
(batteries) or high power density (electrochemical capacitors). There is a growing need, for current and
near-future applications, where both high energy and high power densities are required in the same
material. Pseudocapacitance, a faradaic process involving surface or near surface redox reactions, offers
a means of achieving high energy density at high charge–discharge rates. Here, we focus on the
pseudocapacitive properties of transition metal oxides. First, we introduce pseudocapacitance and
describe its electrochemical features. Then, we review the most relevant pseudocapacitive materials in
aqueous and non-aqueous electrolytes. The major challenges for pseudocapacitive materials along with
a future outlook are detailed at the end.
Broader context
The importance of electrical energy storage will continue to grow as markets for consumer electronics and electrication of transportation expand and energy
storage systems for renewable energy sources begin to emerge. There is a need, particularly with transportation and grid storage applications, where large amounts of energy need to be delivered or accepted quickly, within seconds or minutes. Although carbon based electrochemical capacitors possess the required power density, their relatively low energy density limits their usefulness for these applications. Instead, transition metal oxides that exhibit pseudocapacitance are very attractive. Pseudocapacitance occurs when reversible redox reactions occur at or near the surface of an electrode material and are fast enough so that the device's electrochemical features are those of a carbon-based capacitor, but with signicantly higher capacitances. It is important to recognize that pseudocapacitance
in materials is a relatively new property, with the rst materials identied in the 1970's. Thus, both materials systems and electrochemical characteristics which lead to high energy density at high charge–discharge rates are still being identied. To date, transition metal oxides exhibit the widest range of materials with pseudocapacitive behavior. By selecting the proper transition metal oxide, utilizing the most effective electrode architecture, and analyzing the electrochemical behavior for pseudocapacitive behavior, such materials are expected to become the basis for electrochemical energy storage devices which offer high energy density at high rates.
Introduction
Electrochemical energy storage (EES) in the form of batteries and electrochemical capacitors is widely used for powering the now-ubiquitous portable electronics in our society and for the electrication of the transportation sector. The emerging need to overhaul the power grid in many developed countries combined with the expected rise in global energy needs (arising at least partly from the need to electrify developing countries) over the coming decades have brought another application for EES, the coupling of these technologies with renewable energy sources like solar and wind for powering the electrical grid. While opportunities for EES abound, there are several
challenges for these devices that are rooted primarily in nding materials that are better at both storing and delivering large amounts of energy. These functions would ideally be performed by abundant, non-toxic materials in order to also lower the cost and increase the safety of EES devices in consumer products as
well as in stationary power.
The current success of EES is in large part due to the use of transition metal oxides in one or both electrodes. This review is concerned with transition metal oxide materials that exhibit pseudocapacitance, which arises when reversible redox reactions occur at or near the surface of a material in contact with an electrolyte, or when these reactions are not limited by solid-state ion diffusion. The behavior can exist in both aqueous and non-aqueous electrolytes and can be intrinsic to the material, or extrinsic. The signicant difference between battery and pseudocapacitive materials is that the charging
and discharging behavior of pseudocapacitive materials occurs on the order of seconds and minutes. Thus a strong motivation for studying and developing pseudocapacitance is that it leads to both high energy and high power densities in the same material
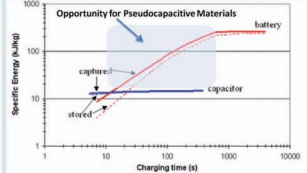
Fig. 1 shows the specic energy vs. charging time plot for an
electric double layer capacitor (EDLC) and a high-rate lithiumion battery.1 This plot clearly demonstrates that a lithium-ion battery optimized for high-power exhibits constant energy density for discharge times >10 minutes. At shorter timescales, this energy decreases due to the various resistive losses within a battery cell, mainly stemming from sluggish electron and ion transport. These resistive losses, particularly at high rates, give rise to heat generation which can lead to serious safety problems such as thermal runaway.2 On the other hand, commercially-available EDLCs exhibit constant energy densities for all timescales but their total stored energy is low. In between the regimes where EDLCs and lithium-ion batteries exhibit their best performance is a time domain (!10 s to 10 minutes) that appears well-suited for the pseudocapacitive materials described in this review.