FDA Makes Homeopathy Remedies ‘Illegal’
The American Food and Drug Administration has reported plans to make homeopathic cures illicit as a major aspect of their crackdown on elective medication.

The FDA intends to focus on all cures that aren't created by Big Pharma, driving them to enroll for a FDA permit.
Dr Mercola reports: "[I]n one singular motion, the FDA has pronounced that for all intents and purposes each and every homeopathic medication available is being sold illicitly," the Alliance for Natural Health USA (ANH USA) composed — and it's not a distortion. Basically, the FDA's direction peruses:
.png)
The FDA at that point expresses that it's proposing "another, chance based authorization approach" and first intends to focus on the "unapproved sedate items named as homeopathic that have the best potential to make chance patients." This incorporates:
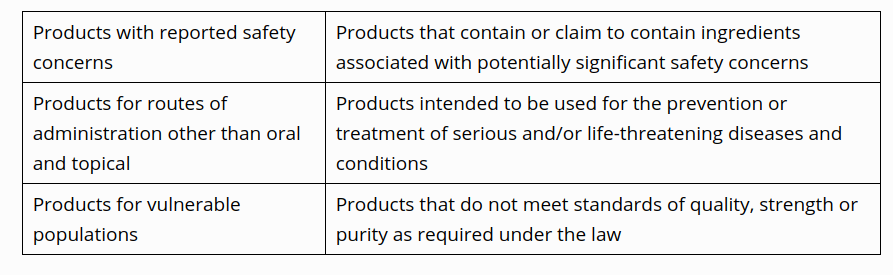.png)
FDA Does About-Face, 'Punishes an Entire Industry'
The draft direction is an entire turnaround from the FDA's earlier position on homeopathic cures, which expressed, under the 1988 Compliance Policy Guide (CPG) 400.400, Conditions Under Which Homeopathic Drugs May be Marketed, they didn't have to get FDA endorsement before going ahead the market. Rather, the fixings in homeopathic cures were to be confirmed by the Homeopathic Pharmacopeia of the United States (HPUS). As supported by law office Venable:
"Basically, the HPUS makes a monograph for homeopathic medications to take after, not at all like the OTC medication monographs that FDA has created for allopathic medications. Likewise, by temperance of a fixing's incorporation in the HPUS, the fixing has just been nearly analyzed and resolved to be protected and successful by the HPCUS [Homeopathic Pharmacopeia Convention of the United States].
Consistence with the HPUS capacities as a premarket audit of security and adequacy in the homeopathic setting. By ordering homeopathic medications as unapproved new medications, the FDA is requiring a moment appearing of wellbeing and adequacy, a stage that is superfluous, also illogical, given that the Agency means to hold homeopathic items to an allopathic standard that is wrong, given the idea of homeopathic items."
Further, it was by the FDA's own particular think choice that homeopathic medications were rejected from previous medication endorsement procedures and expected to be a different class. Venable proceeded:
"The FDA purposely rejected homeopathic medications from both the Drug Efficacy Study Implementation (DESI) audit (the procedure by which all medications affirmed in the vicinity of 1938 and 1962 were reflectively assessed by the FDA for adequacy) and the OTC Drug Review in 1972 (the procedure used to create OTC medication monographs for allopathic medications), choosing rather in the last case to control homeopathic items independently in view of their uniqueness.
By pulling back the CPG and grouping every single homeopathic medication as unapproved drugs subject to FDA sedate endorsement, the FDA is currently requiring higher administrative edges for OTC homeopathic medications than are relevant to allopathic OTC medications, a large number of which are allowed to be sold according to the FDA monograph framework. This move in actuality punishes a whole industry for the Agency's 1972 choice."
FTC Targeted Homeopathy in 2016
The FDA isn't the principal government office to focus on this current hundreds of years' old common medication framework. In a notice recorded in November 2016, the FTC expressed that all together for homeopathic solutions for assert they are successful, their creators must give verification. In the event that no verification is given, the cures must state there is "no logical proof that the item works."
With a specific end goal to not misdirect purchasers, the FTC additionally expressed that homeopathic cures lacking adequate evidence must discuss to shoppers that "the item's cases are construct just in light of speculations of homeopathy from the 1700s that are not acknowledged by most present day medicinal specialists." Dana Ullman, MPH, CCH, one of the main supporters for homeopathy in the U.S., said at the time:
"Considering the long-term security history of homeopathic drugs, it is astounding and notwithstanding stunning that the FTC would consider proposing new controls now.
One really want to ponder who or what is pulling their strings … approaches … regularly result from effective financial powers at play … obviously this administrative organization is disregarding critical logical confirmation, and one must think about whether they are shielding Big Pharma from rivalry more than securing the customer."
Does Homeopathy Work?
In view of the hypothesis that "like cures like," or the Law of Similars, homeopathy was established by German doctor Dr. Samuel Hahnemann. The thought is that wellbeing conditions can be mended by treating a man with minute measurements of a substance that would deliver comparative indications to their wellbeing condition if given in bigger dosages.
Homeopathy's other controlling standard is that of the base measurement, which depends on the start that the more a substance is weakened, the more powerful homeopathic cure it progresses toward becoming, known as the "law of infinitesimals."
Homeopathic cures might be produced using plants, minerals or different substances and are ordinarily directed in pellet frame, broke down under the tongue. Various fascinating examinations propose homeopathy is compelling and alright for an assortment of illnesses, including influenza. As indicated by Ullman:
"The utilization of a homeopathic drug called Oscillococcinum is a case of a cure that can be powerful for some individuals with influenza, however clinical experience proposes that it is best when utilized inside 48 hours of getting influenza side effects. Albeit most homeopathic medications are produced using the plant or mineral kingdom, Oscillococcinum is bizarre in that it is produced using the heart and liver of a duck."
In one examination, about twice the same number of patients who were given Oscillococcinum recouped from this season's cold virus inside 48 hours as those given a fake treatment. Further, Ullman noted:
"An alternate gathering of scientists led a randomized, twofold visually impaired investigation including 372 patients (188 treated with Oscillococcinum and 187 with fake treatment) of both genders, extending in age from 12 to 60, who introduced rectal temperature ≥ 100.4 F, muscle agonies, migraine, or possibly one of the accompanying manifestations: shuddering, chest torment, spine torment, hacking, aggravation of nasal mucosa or feeling of discomfort.
Patients got three containers of Oscillococcinum or fake treatment every day (morning, twelve and night) for three days. The aftereffects of this trial demonstrate a very factually critical distinction between the two gatherings, for what concerns vanishing of side effects following 48 hours (19.2 percent in the Oscillococcinum assemble versus 17.1 percent in the fake treatment gathering) and change in side effects (43.7 percent versus 38.6 percent for placebo)."
Extra research demonstrates homeopathy's promising part in the accompanying conditions:
.png)
FDA Continues to Crack Down on Natural Substances, Protect Big Pharma
The FDA's new push to manage homeopathic items is frightfully reminiscent of their current crackdown on the plant kratom. Leaves from the kratom tree have been utilized for relief from discomfort for a long time, however researchers now know they contain exacerbates that objective the mind comparably to opioids, assuaging torment. It shows up the plant might be more secure than opioids for relief from discomfort and could even go about as an instrument to help those agony from opioid withdrawal.
In November 2017, notwithstanding, instead of focusing on the remedy opioids that are the base of the opioid pandemic, the FDA issued a general wellbeing warning in regards to dangers related with kratom utilize, recommending that its utilization could "grow the opioid scourge." What this comes down to, for a few, is the privilege to pick what type of "prescription" to put in your body. As Dr. Lee Hieb, an orthopedic specialist and past leader of the Association of American Physicians and Surgeons, wrote in WND:
"I'm not here to tout a specific over-the-counter cure, however who gave the FDA add up to capacity to constrain what we can take into our bodies, while basically driving us to be given what they recommend? It's not their concern whether a 'homeopathic cure' is useless.
That is an issue of trade and truth in publicizing. I, for one, need the opportunity to investigate and choose for myself what supplements to take, regardless of whether my supplements eventually do me no great. (Numerous complete a lot of good as Big Pharma knows very well indeed.)"
Individuals have the privilege to pick their treatment, and specialists (a considerable lot of whom confess to recommending fake treatments to their patients) likewise hold the privilege to offer medications they regard valuable, regardless of whether it be homeopathy or another cure.
Concerning the FDA's new draft direction on homeopathic medications, it's endeavoring to dishonor such cures' demonstrated mending powers before their components of activity have even been completely caught on. In the event that you need to express your assessment on the issue, the FDA is tolerating remarks from general society until March 30, 2018.
Congratulations @joshwho2! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOP