How long does the COVID-19 vaccination last in people who are fully vaccinated?
For context this is a question I answered on Quora
Having read all of the pro-vaxx answers on the parent question with zero references to independently verifiable literature I’ve decided to do this question justice by providing a specific answer backed by several references to independently verifiable literature as I do on all of COVID19 related questions that are not anecdotal in nature.
The time specific VE for modRNA boosters is 4–6 months tops for adults but with the last XBB1.5 booster only a couple months before the antigenically divergent JN1 variant became the dominate strain in the winter of 2023–24. For children the time specific VE tends to be even shorter in the range of 2–4 month and sometimes even less than that. Booster recipients develop immune tolerance to the spike protein either between 6 and 9 months or 9 and 12 months after their last booster dose.
Adults
A prior study, published in the New England Journal of Medicine, that extrapolated data from n = 1.25 million vaccine recipients >60 years of age in the Israeli Ministry of Health database, who were eligible for the second booster, found that recipients of the fourth dose initially had lower rates of infection (171 cases per 100K person days), an adjusted rate ratio of 2, compared to the original booster group (340 cases per 100K person days) and an adjusted rate ratio of 1.8, compared to the internal control group (308 cases per 100,000 person days) after four weeks. However, starting at week five and after 8 weeks, the adjusted rate ratio of the fourth dose group declined in comparison to the control group and third dose group to adjusted rate ratios of 1 (i.e. the same rate ratio) for the former and 1.1 for the latter. The authors conclude that: these findings suggest that protection against confirmed infection wanes quickly…The adjusted rate of infection in the eighth week after the fourth dose was very similar to those in the control groups. These findings indicate no (statistically significant) difference.
A prospective observational study conduct among boosted healthcare workers in an Italian hospital (n = 942) who received either COVID19 booster or flu immunization by themselves or in combination, found that 41.5% had SARS-COV-2 infections within a 9 month follow up period (280 days), 82.6% of whom developed symptomatic illness with an average time of less than 5 months (140 days) between their booster dose and infection.
The SIREN Cohort study conducted among vaccinated UK healthcare workers over 18 years of age between September 2021 and February 2022 (n = 19,614) found that the first booster dose had a VE of 63% against infection against the delta variant but this dropped to 32% against infection from the omicron variant within the first two months of its emergence with no additional benefits from the booster after 4 months. Most importantly, the SIREN study found that immunity from a prior infection remained as high as 85% effective against a delta variant infection for 15 months after the primary infection, much higher than the estimated protection odds afforded to recipients of the primary series or booster without a prior infection. With the emergence of omicron this fell to 25% 9 months after the primary infection which while low is still higher than that estimated for the first booster. This study was perhaps one of the first to theorize that mucosal immunity from a prior infection could be a superior route to developing immunity against reinfection and severe disease.
UK vaccine surveillance data for week 23 of June 2023 finds that the monovalent boosters are initially 30% effective against infection, 40% effective against symptomatic disease and 60% effective against hospitalization in the first month. This drops to 20%, 40% and 40% in months 2–3 and 10%, 10% and 20% in months 4–6. 6 months post administration, the monovalent boosters are 0% effective against infection and 0% effective against hospitalization against subvariants BA4, BA5, BQ1 and CH1.1. The bivalent booster does not fair any better producing an initial VE of 30% against infection, 40% against symptomatic disease, and 55% against hospitalization in the first month. This drops to 20%, 40% and 50% in months 2–3 and 10% against infection and symptomatic disease in months 4–6. The bivalent booster VE against hospitalization fairs even worse against XBB1.5 initially starting with a VE of 57% in the first 2–4 weeks but rapidly dropping below 50% in weeks 5–9, dropping to a little more than 25% in weeks 10–14 and dropping to 12% after 15 weeks (3.5 months). The highest number of hospitalization occurred in booster recipients 75+ years of age 3–6 months post administration.
A retrospective population wide study conducted in Austria among previously infected Austrians who had not been vaccinated with non-ModRNA products (n ~4 million) between November 1, 2022 and December 31, 2022, published in The European Journal of Clinical Investigation, found that out of the 69 deaths with COVID that occurred during this interval 31 had received the bivalent booster, 20 had received the original booster only, 11 were unvaccinated and 7 had only received one or two doses of the primary series. There were 8,511 infections among 281,291 recipients bivalent booster recipients (3% of recipients), 37,624 infections among 1.545 million original booster recipients (2.4% of recipients), 22,554 infections among 933,277 recipients of one or two doses of the primary series (2.4% of recipients) and 20,367 infections among 1.225 million unvaccinated persons (1.7% of recipients). From COX proportional hazard ratios, the authors found a relative VE of -24% between the fourth and third dose for COVID mortality and a 17% VE for infections. There were no significant other group differences in COVID-19 mortality, but fewer infections were recorded in the less vaccinated groups (Table 2). A follow up study between January 1, 2023 and June 30, 2023 among recipients of four doses (n = 490,623), three doses (n = 1.352 million), two or one dose (n = 911,896) and the unvaccinated (1.223 million) found that out of 225 deaths with COVID that occurred during this interval 95 had received the bivalent booster, 75 had received the original booster only, 29 were unvaccinated and 26 had only received one or two primary series doses. There were 29,808 new infections among bivalent booster recipients (6.07% of recipients), 80,246 new infections among three dose recipients (5.9% of recipients), 39,156 new infections among primary series recipients (4.3% of recipients), and 24,964 new infections among the unvaccinated (2.04% of recipients). Analyses in 2023 confirm no relative VE for the bivalent booster versus the original booster for COVID-19 mortality (4%, 95% CI: −31 to 29), but show higher risk of infection with a relative VE of −17% (95% CI: −19 to −15) (Table 4). After the follow up study the authors conclude that evidence shows VE peaks at about 3–5 weeks post inoculation but then rapidly declines within a couple of months with ‘no remaining effect beyond 15 weeks was previously reported and fits well to our findings.’ They also found that ‘compared to three vaccine doses, those with fewer or no vaccinations did not differ with regard to COVID-19 mortality but had reduced risk of SARS-CoV-2 infections.’
The test negative case control study conducted in several Eastern European countries (n = 5,165) was missing data on previous infections for nearly half of participants and only enrolled patients hospitalized for severe acute respiratory infections including those unrelated to COVID19. Unsurprisingly, the median age for participants in this study is 64 and 63% have at least one chronic medical condition (i.e. are sick prior to infection) which establishes a clear cohort bias towards older and immunocompromised persons who do not reflect the general demographics of people who have contracted SARS-COV-2 over the past four years (i.e. everyone). Another bias not mentioned until the very end is that test negative controls were about 2.5x more likely to have prior documented infections than patients with COVID19. The seroprevalence of anti-nucleocapsid antibodies and lymphocytes is also extremely low (only 17% have documented prior infections) compared to today. Thus, the study wouldn’t be very informative of estimating the clinical benefit of the shots for young healthy children, adolescents and young adults or immunocompetent persons in general with prior infections. Despite pulling out all the stops to find high mean VE they could not find it past 6 months after the last dose and at 6 months post administration mean VE was already in negligible single digit territory. Averaged over a year, mean VE against hospitalization with severe acute respiratory infection was a measly 15% with a lower limit of -5% (a 5% higher relative risk of infection).
Annual VE was 60% (95% Confidence Interval (CI) 12–82) for a last dose received up to 89 days months prior, 59% (95% CI 31–76) for last dose received 90–179 days prior, 7% (95% CI -29–33) for 180–269 days, and -6% (95% CI -44–22) for 270–364 days. Overall annual VE for last vaccine dose received in the entire previous 365-day period was 15% (95% CI -5–32) (Table 3 and Figure 4). In secondary analyses, both absolute VE and rVE had similar point estimates and trends.
Annualized mean VE against hospitalization was pretty much negligible for the most at risk population: patients over 60 years of age. It is especially concerning for this population because during the course of the study COVID19 cases peaked in the Summer of 2022, more than 6 months after the first booster doses were released in September 2021.
When we limited our analysis to SARI patients ≥ 60 years old, annual VE was 44% (95% CI -33–77) for last dose received up to 89 days prior to onset, 50% (95% CI 7–73) at 90–179 days, -3% (95% CI -51–30) at 180–179 days, and -14% (95% CI -67–22) for those with a last dose 270–364 days before symptom onset. Annual VE for last vaccine received in the previous 365 days was 5% (95% CI - 23–27).
Results in sensitivity analyses for absolute VE and rVE were similar (Table 3, Figure 4)A mean 5% lower relative risk of hospitalization is something that could happen randomly. 5% lower risk is not meaningful. The authors only refer to “waning VE” after 6 months but do not explain the findings of negative VE that they likely obtained from outbreaks in the Summer of 2022 and fall of 2023. Negative VE is immune tolerance found in observational data. The modRNA shot isn’t what is waning in protection; the recipients’ immune system is waning and becoming tolerant of the spike protein. As I explained in a prior answer this coincides with an antibody subclass switch from the promulgation of IgGE, IgG1 and IgG3 anti-spike nabs to IgG4 antibodies that block the former from binding to antigens and stimulating other complement immune processes such as interferon signaling and phagocytosis resulting in disease enhancement in immunocompromised individuals which can also occur as a result of IgG4 antibodies binding to viral surfaces and incorporating them into white blood cells that effectively become hosts for replication. The subclass shift takes at least a few months to occur which may explain why we observe initially high VE against infection and symptoms in the first two months that rapidly declines between months 3-4 post administration, becomes negligible between months 4-6 and results in an observation of negative VE against infection after 6 months (well before the next annual booster rollout).
A systematic review published in the JAMA Network journal on May 3, analyzed 40 studies estimating vaccine effectiveness (VE) over time against laboratory-confirmed COVID-19 infection and symptomatic disease. The studies were selected from 799 original articles, 149 reviews published in peer-reviewed journals, and 35 preprints. The review found that the vaccine effectiveness of a primary vaccination cycle against the Omicron infection and symptomatic disease was lower than 20 percent at 6 months from the administration of the last dose. Booster doses restored vaccine effectiveness to levels similar to those seen after administration of the primacy cycle dose. However, nine months after the booster dose, vaccine effectiveness against Omicron was found to be lower than 30 percent against infection and symptomatic disease.
“The half-life of VE against symptomatic infection was estimated to be 87 days for Omicron compared with 316 days for Delta. Similar waning rates of VE were found for different age segments of the population.”
“These findings suggest that the effectiveness of COVID-19 vaccines against laboratory-confirmed Omicron or Delta infection and symptomatic disease rapidly wanes over time after the primary vaccination cycle and booster dose,” the study said. “Putting together the bulk of available evidence on the waning of VE over time against COVID-19 variants has crucial implications for future interventions and vaccination programs.”
87 days is less than 3 months.
A prospective cohort study of healthcare workers without prior covid infections (n = 11,176) , published in the New England Journal of Medicine, found that the second booster VE against omicron infection declined from 52%, during the first 5 weeks, to -2% at 15–26 weeks (3–6 months). A concurrent study of neutralizing antibodies in boosted healthcare workers (n = 6,113) found that IgG antibody levels peaked at week 4 post administration and gradually declined to baseline levels prior to the second booster.
A study of long-term booster effectiveness conducted by Chemaitelly et al., found that the booster is initially 41% effective against infection relative to the primary series but rapidly declined to 14.4% VE in month 6 and became negative thereafter. The booster was found to have a VE of -20.3% against subvariants BA4, BA5 and BA2.75 suggesting immune tolerance to the spike protein of these mutants.
Tseng, 2022 et al., using a test negative case control design to study outcomes for covid patients within the Kaiser Permanente Southern California Health System (n = 4.7 million) found that the first booster VE against BA1 infection dropped from 85%, 14–30 days post vaccination, to 55% after 5 months. Booster VE against BA2 infection fell from 61% to -25% during the same periods. VE against BA.2.12.1 infection dropped from 82.7% to -26.8% over the same time periods. VE against BA4 infection dropped from 72.6% to -16.4% over the same time periods. VE against BA5 infection fell from 90.6% to -18% over the same time periods. The second booster VE against BA2 dropped from 64.3% 14–30 days post vaccination to 17.3% after 3 months. VE against BA.2.12.1 dropped from 64.4% to 14% over the same time periods. VE against BA4 dropped from 75.7% to 6.3% over the same time period. VE against BA5 fell from 30.8% to 5% over the same time period.
“While 3-dose VE against BA.1 infection was high and waned slowly, VE against BA.2, BA.2.12.1, BA.4, and BA.5 infection was initially moderate to high (61.0%-90.6% 14-30 days post third dose) and waned rapidly. The 4-dose VE against infection with BA.2, BA.2.12.1, and BA.4 ranged between 64.3%-75.7%, and was low (30.8%) against BA.5 14-30 days post fourth dose, disappearing beyond 90 days for all subvariants.”
A Danish cohort study reports an initial VE against infection of 55.2% for Pfizer and 36.7% for Moderna after the booster but quickly declines within a few months to 16.1%, 31–60 days after the Pfizer booster, and 30%, 31–60 days after the Moderna booster. Between 61–90 days, the Pfizer booster has a VE of 9.8% while the Moderna booster has a VE of 4.2%. We reach negative VE territory between 91–150 days after the booster.
Tamandjou et al., used a test negative study to estimate VE for the second booster compared to the first booster in symptomatic patients 60 years or older in France between March and October 2022 (n= 933,491). They found that the protection against symptomatic disease offered by the second booster was inferior to the protection offered by the first booster during the same time period after administration with a VE of 39% 7–30 days post administration of the second booster compared to a VE of 64% for the same period after the first booster. The VE of the second booster dropped to 8% after 3–4 months while the VE of the first booster dropped to 33% after the same time period. VE after the second booster fell below zero between months 4 and 5 while VE of the first booster during the same time period did not.
Note: This is what immune tolerance looks like.
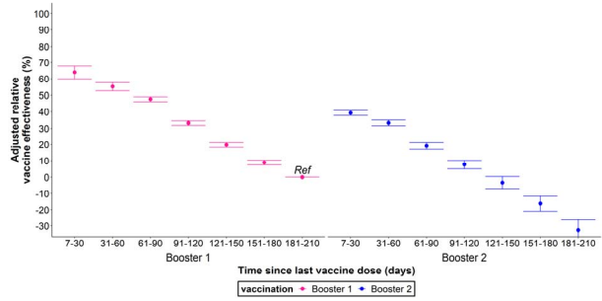
The Open label COVE trial (n = 29,035) conducted from 2021–23 found that the Moderna booster reduced the incidence of omicron BA.1 disease by 56% after the 14 day case counting window, which rapidly declined to 38% VE 60 days (2 months) after the booster and a clinically insignificant VE of 4% 120 days (4 months) after the booster.
A CDC test negative case control study (n = 7,677)conducted among immunocompetent expectant mothers between June 2022 and August 2023 found that 57% of the COVID19 cases (n = 946) among participants occurred in vaxxed expectant mothers while 43% occurred in unvaxxed expectant mothers. Booster VE against COVID19 emergency department/urgent care admissions was estimated to be 28% (with a lower limit of 11%) if received less than 6 months prior to pregnancy and 6% (with a lower limit of -11% I.e. WORTHLESS) if the last booster was received 6 months or more prior to pregnancy. Notably, this study does not account for natural immunity from prior infection which every westerner has acquired by now and lasts much longer than 6 months and includes all possible emergency department/urgent care admissions that occurred simultaneously with a SARS-COV-2 infections regardless of whether the infection was the cause of the admission. If both confounding variables were controlled in this study estimated time specific VE would be even shorter.
A population-based test-negative case control study of XBB1.5 booster VE, conducted among adults 60 years of age or older in a Quebec hospital between October 2023 and August 2024 (n = 114,005) found that XBB1.5 VE against COVID-19 hospitalization, compared to participants with only prior monovalent/bivalent boosters, was a mean 30% over a ten month period. Mean VE against hospitalization was 54% when XBB1.5 was the dominant sub-variant but fell to 23% when JN1 became the dominate variant and 0% against the KP variants that emerged in May 2024. Stratification of results by time since XBB1.5 booster administration revealed statistically significant reduced risk of COVID-19 hospitalization was only observed in the first 4 months after the booster for each sub variant. During the period where JN1 became the dominate variant, VE against hospitalization fell to 17% 5–6 months post administration and -5% (5% higher risk of hospitalization) 7 months post administration. Stratified by calendar time, XBB1.5 boosters given in October 2023 reduced risk of COVID-19 hospitalization by 50% in the first 2 months (November and December 2023), by only 30% in January 2024 and provided no reduced risk of COVID-19 hospitalization between February and August 2024 (I.e. the end of the winter wave and start of the summer wave).
Children
Using the UK’s OpenSAFELY-TPP database, Hulme and Colleagues conducted a retrospective matched cohort study of VE for adolescent recipients between 12 and 15 years of age (n = 820,926) and found that the first dose only lowered the incidence of positive SARS-CoV-2, compared to the unvaxxed controls, for 15 weeks (3.4 months).
By 15 weeks the cumulative incidence of positive SARS-CoV-2 tests was similar in the first dose and unvaccinated groups. The 20-week risks per 10,000 were 1,961 (95% CI 1,932-1,990) and 1,979 (1,950-2,008) in the vaccinated and unvaccinated groups respectively.
Similarly, adolescent recipients of the second dose only had a significantly lower incidence of positive tests compared to recipients of only the first dose for 14 weeks (3.2 months).
The incidence of positive SARS-CoV-2 test after the second dose declined between 10 days and 6 weeks after vaccination, then increased (Figure 1). By 14 weeks the cumulative incidence of positive SARS-CoV-2 test was similar in the second and single dose groups. The 20-week risks per 10,000 were 850 (95% CI 802-899) and 898 (861-935) after the second and single dose respectively.
Adolescents in the UK became eligible for the first dose in September 2021, 2 months prior to the emergence of omicron which didn’t become the predominate variant for another couple months so they are still measuring VE against the Delta variant for most of that 20 week risk window.
A study of Vaccine efficacy among vaccinated children 5–11 years of age within New York (n = 365,502 ) found that VE against infection drops from around 45–55% to about 11% VE within a few weeks. The incidence rate ratio for infection compared to unvaccinated children 5–11 years of age fell from 3.1 on December 13, 2021 to 1.1 (statistical insignificance) by January 24, 2022 (42 days). VE against infection for Children 12–17 years of age dropped from 76% to 46% within the same 42 day time period.
42 days is 6 weeks. Children were exposed to a higher risk of several different autoimmune disorders for 6 weeks of clinically meaningful relative risk reduction.
A test-negative, case-control analysis, published in the Journal of American Medical Association, found that the VE of the primary series dropped to zero against omicron three months after it was administered to children and adolescents. The analysis examined 43,209 negative test controls and 30,999 positive test cases for children 5–11 years of age (n = 74, 208) as well as 25,471 negative test controls and 22,273 positive test cases for adolescents between 12–15 years of age (n = 47,744) from 6,879 test sites across the U.S. between December 2021 and February 2022. VE against symptomatic omicron infection was estimated at 60.1% for 5–11-year-olds and 59.5% for 12–15-year-olds 2–4 weeks after the second dose of the Pfizer primary series. VE rapidly declined to 28.9% for 5–11-year-olds and 16.6% after 2 months and dropped to almost 0 VE 3 months after the second dose for both cohorts. A different analysis from the same test platform reported VE of 42% against symptomatic omicron infection 2–4 weeks after the second Pfizer dose which dropped to 0 three months after the second Pfizer dose.
A Pfizer sponsored test-negative case-control study, published in the Journal of the American Medical Association, of children (n =24,261) between 6 months to 4 years of age found an overall VE of 33% against symptomatic infection for the combined two and three shot group but a higher risk of symptomatic infection after three shots compared to two shots within the 90 days window of the study. The three-shot group VE against symptomatic infection was only 12% compared to the unvaccinated group (i.e. within the confidence interval and basically negligible) within the 90 days window of the study. It should be remembered that three 3 microgram doses is considered a standard for children and only 2 is considered an incomplete series; thus, completely vaccinated children between 6 months and 4 years of age have negligible protection compared to unvaccinated children.
The CDC’s latest PR observational study attempt to save the VE narrative, which has been restricted to severe disease and hospitalization reduction, incidentally confirms that “robust protection” of modRNA therapy advertised against hospitalization has about a 4 month lifespan if that.
Mean VE against hospitalization is estimated at 25% between 4 months to 364 days out with the lower limit at - 9%. The anytime within the previous year stipulation seemed to improve the mean VE against hospitalization to 38% with a lower limit of 15% which to an immunocompetent child or adolescent with no comorbidities, sufficient Vitamin D serum levels and/or nucleocapsid antibodies and memory cells from prior omicron infection(s) is completely meaningless and not worth the several fold higher relative risk of cardiomyopathy.
Compared to every other “vaccine” that’s ever been produced and mandated this is third rate garbage and the bottom of the barrel of third rate garbage at that. If you are still trying to force this third rate garbage on children and adults you are not only a totalitarian shitbag you’re also a corporate cocksucking POS carrying water for a fraudulent psychopathic multinational conglomerate that has committed fraud and colluded with so called regulators dozens of times with a rap sheet longer than a CVS receipt. As I pointed out in a prior post you need anti-nucleocapsid Immunoglobulin A induced mucosal immunity to prevent infection at the actual site of infection. Circulating IgA does not enter mucosal secretions in the upper respiratory tract and neither do IgG induced nabs in the lymphatic system. This is why this third rate vaccine, if you can even call it that, only has a time specific VE of 3 to 6 months. Even the annual flu shot has a higher time specific VE (6–8 months against identical strains) than this supposed blockbuster noble prizing famed product. Unlike traditional vaccines that take the form of an inactive or live weakened antigen, modRNA therapies fail to produce long lived plasma cells that maintain hormonal response for more than a few months.
🚨 URGENT CALL TO ACTION 🚨
Hey friends! 👋 Let's dive into this fascinating topic about vaccine effectiveness (VE) against symptomatic omicron infection.
Did you know that studies have shown VE to be as low as 12% for children aged 6 months to 4 years, with the three-shot group actually having a higher risk of symptomatic infection compared to those who only received two shots? 🤯
And get this - according to the CDC's latest observational study, "robust protection" against hospitalization from modRNA therapy has a mere 4-month lifespan! 🕰️
But here's the thing: even with these concerning findings, we can still make informed decisions about our health and well-being. 💡
So, let's discuss this further in the comments below! What are your thoughts on vaccine effectiveness? Have you or someone you know experienced anything like this?
And don't forget to show some love to our wonderful community by voting for our witness 'xpilar.witness' by going to https://steemitwallet.com/~witnesses. Your support means the world to us, and it will help us continue contributing to the growth and success of Steem! 🙏
That is one corporate funded study. There are 20 summarized here. If you removed the asymmetrical case counting window vaxx bias and healthy vacinee bias initial VE would be much closer to zero and negative VE i.e. immune tolerance would set in much quicker.
Oh dear, this is too difficult to understand for me. 🤐
Let's see... Perhaps you should wait if the bot comes back and is willing to start a chat with you?
.jpg)
Let's comment and make people smile starting today.