What the 2022 Nobel Prize in Chemistry was awarded for and why it is important
In 2022, the Nobel Prize in Chemistry was awarded to: American chemist Barry Sharpless, who first introduced the so-called "click chemistry", Danish scientist Morten Meldal, who developed an atomic reaction for it, and American chemist Carolyn Bertozzi, who transferred the method to living cells.
Formulation of the problem
For most people, chemistry is a difficult science. However, I learned about it from those around me, since for me chemistry has always been one of those subjects that came very easily to me. Chemistry was very easy for me at school, and that is why I decided to study it at university. This was a rational approach to choosing a profession.
When your life is closely connected with something, then you even involuntarily take an interest in it more widely. It's the same with me, I'm always interested to find out what new things modern chemists have invented. But the invention for which the 2022 Nobel Prize in Chemistry was awarded really impressed me. It has already changed our lives. We just haven't felt it yet.
While studying at the university, I learned that all the clear rules by which some substances interact with others are not really so clear. The same reaction can give different products under different conditions, no reaction occurs in 100% yield, there is always an unreacted residue and by-products. In a word, not everything is so simple. And the more complex substances you want to get, the more complicated things become.
At the chemical plant where my career began, chlorine and caustic soda were produced from ordinary table salt. These are all very simple substances, for example, salt is NaCl, chlorine is Cl2, and caustic soda is NaOH. Each molecule of these three substances consists of only two atoms. However, despite such simple substances, in order to obtain the desired products from table salt, it was necessary to develop a technological process consisting of 17 stages, to spend many megawatts of electricity, gigacalories of steam, thousands of cubic meters of water, etc. every day.
Now imagine that we need to get a more complex substance. This usually requires many steps. Each stage will be accompanied by the formation of by-products, each stage requires certain conditions: high pressure or temperature, the presence of a catalyst, etc. The more complex a substance is, the more difficult it is to obtain it. But the main thing is that with each new stage, the cost of obtaining such a substance grows exponentially.
Now imagine that we need to get some biological substance. If you have opened a textbook on biochemistry, you know that there are practically no simple substances in living organisms. Moreover, biological compounds are so complex that it is impossible or irrational to obtain them artificially.
However, we need to obtain precisely such complex substances. This is necessary for medicine and pharmacology. They are needed to treat many diseases, they are needed to improve our lives.
What is "click chemistry"
Barry Sharpless was looking for a way to simplify complex synthesis. His idea was modular synthesis, which would create a wide variety of products from simple raw materials. Sharpless and his colleagues call this process modular, because the elements simply "connect" to each other. This is how he laid the foundations of "click chemistry".
Scientists have formulated criteria that must be met by reactions and starting materials for proper modular synthesis:
- chemical reactions must be efficient,
- take place in the presence of oxygen and water, without special solvents, to make them as versatile as possible;
- starting materials should be easily accessible, and by-products should be easily separated.
What did the scientists actually propose? Imagine two molecules:

In order to connect them, it is necessary to achieve certain conditions, for example, extreme pressures or temperatures. Each degree of increased temperature or megapascal of excess pressure is a huge expense on an industrial scale. However, the process can be greatly simplified by attaching these molecules with "something":
Following this approach, you can get a molecule of any complexity.
How to connect molecules?
Sharpless and his colleagues proposed a number of reactions that could be used to connect molecules. Among them was a reaction studied by the Danish chemist Morten Meldal. He found out that the azide-alkyne cycloaddition can be made much more efficient in the presence of a copper catalyst. In this case, high temperatures are no longer needed, and the reaction will proceed almost by itself and with a high yield.
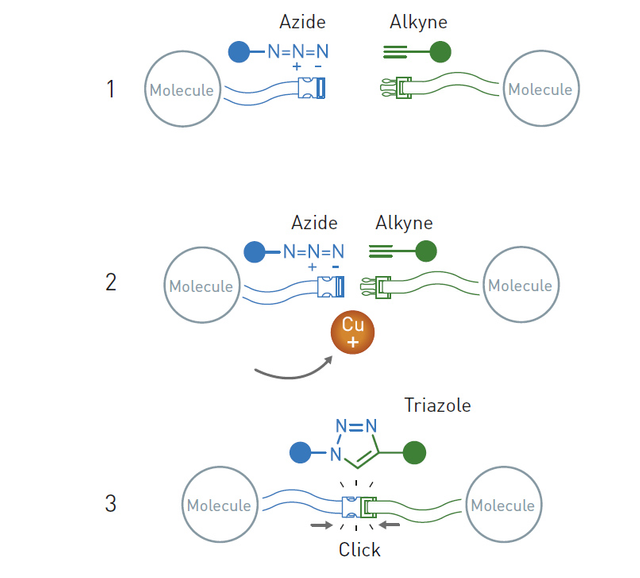
Thanks to this reaction, it is possible to "assemble" more and more complex designs. Today, chemical synthesis is based on this reaction, which is necessary in particular in the production of medicines, plastics and countless other technical, medical and chemical materials.
American chemist Carolyn Bertozzi expanded the basic reaction of "click chemistry", adapting it to living cells. To do this, she had to invent a way to conduct the reaction without a toxic copper catalyst. At the same time, the reaction should be equally effective. She succeeded in this thanks to the use of a ring-shaped alkyne. This made it possible, for example, to attach fluorescent marker proteins to cellular substances.
Carolyn Bertozzi added to the well-known reaction of Staudinger synthesis, thanks to which it can now be used to "couple" molecules directly in the cellular environment. Now reactions of functional groups have become possible, which are so selective that they can bind molecules even in a highly dynamic and complex biological environment.
Conclusions
In my opinion, a revolution has taken place in chemistry. It is hard to even imagine what will become available to us now. Potentially, it will become possible to produce medicines that we could not even dream of until now. Moreover, it seems that some medicines or vital substances can be produced directly in living cells.
In the coming decade, we should expect breakthroughs in the treatment of many ailments.