Antineoplastons: A brief meta-analysis on new controversial cancer treatment, free of Big-Pharma Bias.

Introduction
Antineoplastons are peptides and amino acid derivatives, discovered by Dr R.S Burzynski, M.D, Ph.D. in 1967.
Dr. Burzynski first identified naturally occurring peptides in the human body that control cancer growth. He observed that cancer patients typically had a deficiency of certain peptides in their blood as compared to healthy individuals. According to Dr. Burzynski, Antineoplastons are components of a biochemical defence system that controls cancer without destroying normal cells.
Chemically, the Antineoplastons include peptides, amino acid derivatives and organic acids. They occur naturally in blood and urine and they are reproduced synthetically for medicinal use. The name of Antineoplastons is derived from their functions in controlling neoplastic, or cancerous, cells (http://www.burzynskiclinic.com/what-are-antineoplastons.html). Antineoplaston A10, identified as 3-phenylacetylamino-2,6-piperidinedione, was the first to be synthesized (http://allnutritionals.com/natural-products/antineoplastons.php).
These Antineoplastons are small protein fragments, or peptides, produced in the liver. They block the ability of cancer cells to divide by turning off genetic signals that stimulate cancer cell growth and by turning on genes that prevent growth and even cause apoptosis. They are part of our natural defenses against cancer. When production of Antineoplastons decreases, due to age or other factors, cancer results.
Dr. Burzynski discovered Antineoplastons as a young physician and researcher in his native country, Poland in the 1960s. He observed the presence of certain peptides in the urine of healthy individuals that were absent in cancer patients, he then isolated these peptides and found that in in-vitro studies, they inhibited cancer cell growth. In 1970, Dr. Burzynski immigrated to Houston to continue his research, working first at Baylor College of Medicine; he discovered how to synthesize Antineoplastons and has been using them, orally and parenteraly, to successfully treat tens of thousands of patients over the last 27 years with remarkable success (http://www.afountainofyouth.org/stop-cancer/the-unknown-cancer-cure/).
Dr.Burzynski believes that a variety of Antineoplastons are present naturally in the tissue and interstitial fluids of healthy individuals, but that, possibly as a consequence of cachexia ( metabolic process that results in physical wasting of the human body), cancer patients excrete excessive amounts of these chemical entities in the urine, resulting in decreased circulating levels of these vital Antineoplastons. He states that treatment with Antineoplastons reduces the amount of endogenous Antineoplastons excreted, and that excretion of Antineoplastons decreases with tumor regression. Dr.Burzynski hypothesizes that Antineoplastons may act by interfering with the action of certain enzyme complexes (i.e methylation complex isozymes) that allow malignant cells to gain a growth advantage over normal cells. He has also suggested that Antineoplastons may interact directly with DNA.
(http://www.princeton.edu/~ota/disk2/1990/9044/904407.PDF). Authors note- It has come to my attention that certain medical authorities have tried their utmost best to discredit this novel therapy, if reviewing medical journals or trials stemming from the Mayo clinic please be aware of the dosing regimens administered, small scale trials are performed in such a manner as to cause edema and electrolyte dyscrasies in all test subjects .
Mechanism of action
Antineoplastons act as “molecular switches”, which turn off essential life processes in abnormal cells and force them to die via apoptosis. While they trigger the death of cancer cells, they do not inhibit normal cell growth. They specifically target cancer cells without harming healthy cells.
It is generally known that the cancerous process results from increased activity of oncogenes and decreased expression of tumour suppressor genes. Antineoplastons activate tumour suppressor genes whilst concurrently deactivating oncogenes restoring the proper balance in gene expression (http://www.burzynskiclinic.com/what-are-antineoplastons.html).
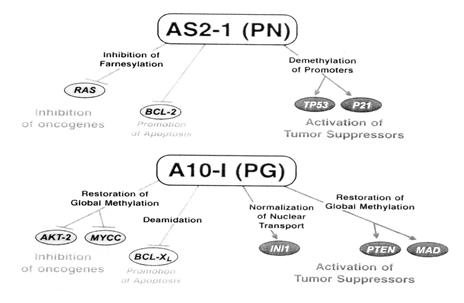
Cancer results from the increased activity of oncogenes and decreased expression of tumour suppressor genes. The proposed mechanism of action of Antineoplastons from laboratory studies is via multiple targets including AKT/PTEN, RAS, p53, p21, MYCC and apoptosis pathways. PN (sodium phenylacetate), the active ingredient of antineoplaston AS2-1, inhibits farnesylation of protein p21 of the RAS oncogene, inhibits RAS and BCL-2, and activates the tumour suppressor genes TP53 and p21 through demethylation of their promoters. PG (phenylacetylglutaminate), the main ingredient of antineoplaston A10-I, restores global methylation of DNA, inhibits the oncogenes AKT2 and MYCC, activates the tumour suppressor genes PTEN and MAD and restores activity of the mutated INI1 protein through normalization of nuclear transport. Both PN and PG promote apoptosis: PN through inhibition of BCL-2 and PG through deamidation of the BCL-XL protein (Burzynski Clinic, 9432 Katy Freeway, Suite 200, Houston, TX 77055). This is reinforced by W.Robert Hudgins et al in the article “Cytostatic activity of phenylacetate and derivatives against tumor cells”. They also state that phenylacetate (an antineoplaston) and its analogues analogs appear to represent a new class of pleiotropic growth regulators that may alter tumor cell biology by affecting gene expression at both the transcriptional and post-transcriptional levels.
It has been shown that aromatic fatty acids, such as, phenyacetate, interfere with protein post-translational processing by inhibiting the MVA pathway of cholesterol synthesis.
MVA is a precursor of several isopentenyl moieties required for progression through the cell cycle, and of prenyl groups that modify a small set of critical proteins. The latter
include plasma membrane G and G-like proteins (e.g. RAS) involved in mitogenic signal transduction and nuclear envelope lamins that play a key role in mitosis. The aromatic fatty acids can conjugate with coenzyme-A, enter the pathway to chain elongation, and interfere with lipid metabolism. Compounds such as phenylacetate can assume a conformation resembling mevalonate pyrophosphate and inhibit MVA utilization specifically. Phenylacetate activity against poorly differentiated tissues is associated with inhibition of MVA decarboxylation and a decline in protein isoprenylation (Hudgins W.R et al, 1995).
Structure activity relationships (SAR’s)
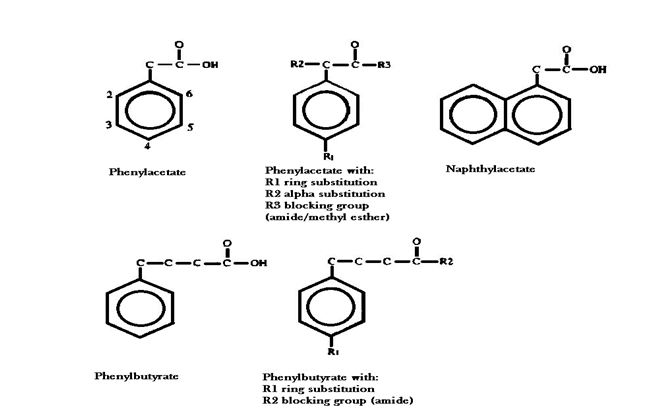
W.Robert Hudgins et al state in the article “Cytostatic activity of phenylacetate and derivatives against tumor cells” that similarly to phenylacetate, derivatives containing α-carbon or ring substitutions induced cytostasis and phenotypic reversion at non-toxic concentrations. Potency was correlated with the degree of calculated lipophilicity of the aromatic fatty acid, and the extent of inhibition of protein prenylation (Hudgins W.R et al, 1995).
This can be explained by the pharmacokinetic principle that states that lipophilicity of a chemical entity is directly proportional to its bio-permeability. W.Robert Hudgins et al discuss this possibility; “One possible function of increasing lipophilicity is an increasing ease with which aromatic fatty acids can enter into and cross the plasma membrane as well as the membranes of other organelles” (Hudgins W.R et al, 1995).
It has been known since 1906 that long chain aromatic fatty acids are subject to fatty acid metabolism and can be β-oxidized repeatedly, but the aromatic ring itself resists oxidation. Consequently, phenylacetate is metabolically resistant, having a half-life in a patient of more than 4 hours, and its analogue, phenylbutyrate, a half-life of l to 2 hours compared with non-aromatic, butyric acid, which has a half-life of only 6 minutes. Preserving the aromatic nucleus and the carboxyl group while making other modifications to phenylacetate might provide more potent analogues of the parent compound (Hudgins W.R et al, 1995).
Compared with derivatives containing ring or α-carbon substitutions, those with blocked carboxyl groups are less effective, for example, the methyl ester of phenylacetate is about half as active as the free acid. The amide forms are also less active than the parent compounds as seen in the research done by W.Robert Hudgins et al; the amide forms of phenylacetate and phenylbutyrate, in which the carboxylic group is blocked, were shown to be less cytostatic in comparison to the parental compounds and failed to induce cell differentiation (Hudgins W.R et al, 1995).
The study done by W.Robert Hudgins et al shows that halogen substitution para to the alkylcarboxyl group increases potency more than that in the ortho position, by a factor of two, suggesting that orientation of the hydrophobic substituent may be important.
At the para position, chlorine had twice the impact on efficacy of the methyl group despite nearly equal contributions to CLOGP, indicating that electronegativity may affect
growth inhibitory interactions. While α-ethylphenylacetic acid, in which the carboxyl group is crowded by the adjacent ethyl group, was more potent than the parent
compound, the slightly more lipophilic analog α-methoxyphenylacetic acid was shown to be less active. Other parameters such as addition of an aromatic ring to phenylacetate, or
an increase in the distance between the aromatic nucleus and the carboxyl group did not cause anomalous enhancement or interference in biological activity (naphthylacetate
and phenylbutyrate were about as active as would be expected on the basis of their lipophilicity). Selectivity was reduced for chlorophenylbutyrate and iodophenylbutyrate,
but not for phenylbutyrate or for the p-oxidation product of chlorophenylbutyrate, chlorophenylacetate. It was postulated that g-oxidation intermediates of some of the substituted phenylbutyrates are active and less selective for tumor cells.
Moreover, phenylacetylglutamine had no detectable effect on cell growth and maturation therefore indicating that a free carboxyl group may be essential for some aspects of the antitumor activity of phenylacetate and its derivatives (Hudgins W.R et al, 1995).
In a study performed by Xun Li et al called “Novel aminopeptidase N inhibitors derived from antineoplaston AS2–5” the following SAR’s were identified in relation to the parent compound depicted below and focused on its ability to inhibit the enzyme APN: 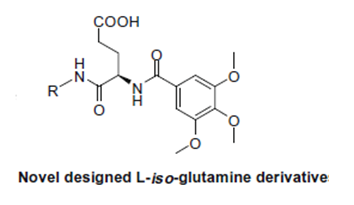
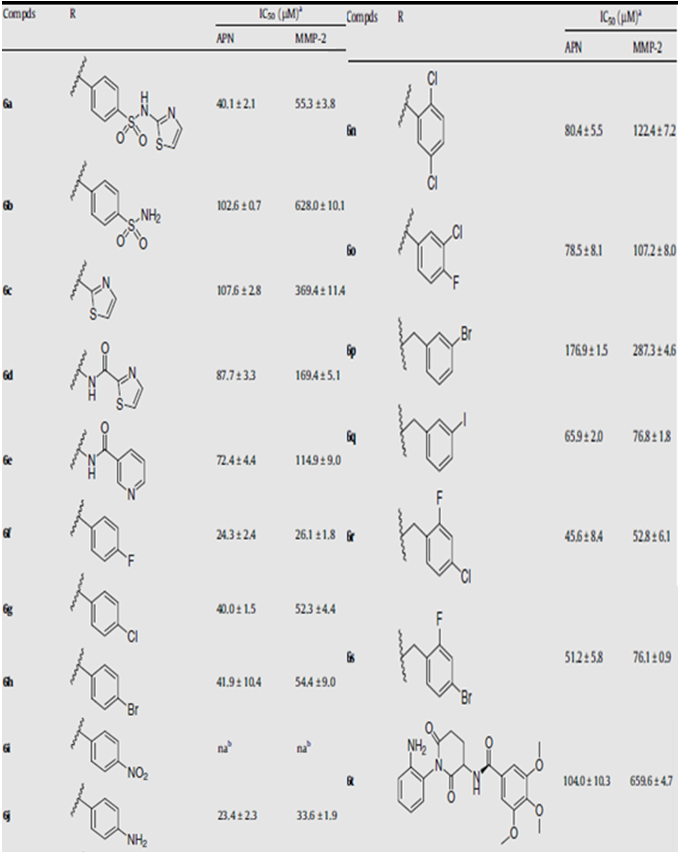
• Compounds with phenylamine groups as their hydrophobic side chains show the best inhibitory activity towards the enzyme APN
• Compounds with aromatic side chains have better activities compared to compounds with aliphatic side chains (this might be due to the p system of the aromatic ring enhancing the interaction with the hydrophobic region of the APN enzyme)
• Substitution on the aromatic ring also has an impact on bioactivity. Phenyl sulfonamide and amide groups are directly involved with APN inhibition (this might be due to the formation of a hydrogen bond with the enzyme backbone, thus stabilizing the binding between the compound and the enzyme).
• Replacing the five-membered thiazole with a six-membered pyridinyl ring produces slightly improved potency.
• Compounds with mono-substituents at the para-position in the aromatic ring (refer to compounds 6f–k), to some extent, due to the electron- withdrawing property of the para-substituent, have a negative effect on APN inhibitory activities. For example, the introduction of amino group (6j) displays the highest activity, the halogen group (6f–h) thereafter; followed by the carboxyl group (6k).The nitro group (6i) presented the least activity having no APN and MMP-2 inhibitory activity. Moreover, as compared with fluoro, chloro and bromo substitutions, it seems that the increased substituent volume may result in impaired activity, suggesting there is a space requirement in the APN binding pockets to accommodate the suitable substituents. The same rule can also be applied to compound 6t.
• As compared with the halogen-substituted compounds, generally speaking, di-substitution at the phenyl moiety (6m–o) moderately impairs the inhibitory activity in comparison to the monosubstituted counterparts (6f–h). However, if you change the phenyl ring into benzyl moiety, the reverse rule can be observed. That is to say, di-substitution at the benzyl moiety (6r and 6s) shows better inhibitory activity compared with the mono-substituted compounds, 6p and 6q.
• Compound 6l, with a hydroxyl group ortho- to the phenyl carboxyl core (at the 3- and 4-positions, respectively), is slightly more potent than 6k, suggesting that hydroxyl group substitution is positively related to inhibitory activity by effectively forming a hydrogen bond with the residue of the APN enzyme
Xun Li et al, “Novel aminopeptidase N inhibitors derived from antineoplaston AS2–5 (Part I)”, Bioorganic & Medicinal Chemistry, 17 (2009) 3053–3060.
Toxicity
The general adverse effects of the formulations A10 and AS2-1 include easily treated abnormalities in plasma electrolytes. Uncommon adverse effects possibly related to antineoplastons include skin rash, somnolence, weakness, nausea, vomiting, headaches, slurred speech, confusion, fever and fluid retention. Adverse effects are reversed on temporary discontinuation of drug or upon dose reduction (Burzynski S.R et al, 1999, pp. 1-10).
The serious side effects of antineoplastons tend to be reversible hyperpyrexia, granulocytopenia and anemia (Stanislaw R. Burzynski et al, 2005, pg 168-177).
Efficacy
It is important to note that the large majority of results of clinical trials done on antineoplastons have been done with patients suffering from different kinds of brain cancer. The prognosis of brain cancer varies based on the type of cancer. Medulloblastoma has a good prognosis if treated with a combination of chemotherapy, radiotherapy, and surgical resection whilst glioblastoma multiforme has a median survival of only 12 months even with aggressive chemoradiotherapy and surgery. Brainstem gliomas have the poorest prognosis of any form of brain cancer, with most patients dying within a year.
“Phase I studies with phenylacetate confirmed that millimolar levels can be achieved in the plasma and cerebrospinal fluid with no significant toxicities, and result in clinical improvement in patients with high-grade gliomas and hormone-refractory prostate cancer” (Hudgins W.R et al, 1995).
In a phase II clinical trial done by Burzynski S.R et al, the results showed that antineoplastons A10 and AS2-1 eliminated or substantially reduced tumors in 44% of patients with brain tumors. Of the 36 patients evaluated in the study, nine had a complete response, seven a partial response, and 12 stable disease. Progressive disease occurred in only eight patients. 15 patients are alive today, 86.5% of them for over 3 years from the beginning of treatment. It is important to note that complete and partial responses were documented in glioblastoma multiforme, astrocytoma, oligodendroglioma, mixed glioma, medulloblastoma, and malignant meningioma, some of these being pathologies with very poor prognosis. In conclusion antineoplaston therapy produced complete or partial responses in 16 of 36 (44%) patients with brain tumors. In comparison to standard treatment, antineoplaston therapy is associated with prolonged survival time and prolonged time to disease progression (Burzynski S.R et al, 1999, pp. 1-10).
In yet another phase II clinical trial the efficacy of antineoplastons in low-grade glioma, brain stem glioma, high-grade glioma, adenocarcinoma of the colon, and hepatocellular carcinoma was portrayed. The best results were observed in children with low-grade glioma, where 74% of patients obtained an objective response, and in patients with adenocarcinoma of the colon with liver metastases whose survival rate was more than 5
years for 91% of patients versus 39% for patients in control groups receiving chemotherapy (Burzynski S.R et al, 2004, pg 47-58).
In a study done by Burzynski S.R et al called “Targeted Therapy with Antineoplastons A10 and AS2-1 of High-Grade, Recurrent, and Progressive Brainstem Glioma”, the obtained results were as follows. The overall survival at 2 and 5 years was 39% and 22%, respectively, and maximum survival was more than 17 years for a patient with anaplastic astrocytoma and more than 5 years for a patient with glioblastoma. Progression-free survival at 6 months was 39%. Complete response was achieved in 11%, partial response in 11%, stable disease in 39%, and progressive disease in 39% of patients. In conclusion antineoplastons contributed to more than a 5 year survival in recurrent diffuse intrinsic glioblastomas and anaplastic astrocytomas of the brainstem (Burzynski S.R et al, 2006, pg 40-47).
Lastly the results from a phase II clinical trial conducted under the heading “Long-term Survival of High-Risk Pediatric Patients With Primitive Neuroectodermal Tumors Treated With Antineoplastons A10 and AS2-1” shall be discussed. Primitive neuroectodermal tumors (PNET’s) are usually successfully treated with craniospinal radiation and chemotherapy; however, difficulties with standard treatment can be encountered in very young children, in adult patients at high risk of complications from standard treatment, and in patients with recurrent tumors. Thirteen children, either with recurrent disease or classified as high risk, were treated in this phase II study with antineoplastons. The median age of patients was 5 years, 7 months (range, 1-11 years). Medulloblastoma was diagnosed in 8 patients, pineoblastoma in 3 patients, and other PNET in 2 patients. Previous treatments included surgery in 12 patients (1 had a biopsy only, suboccipital craniotomy), chemotherapy in 6 patients, and radiation therapy in 6 patients. Six patients had not received prior chemotherapy or radiation. The treatment consisted of intravenous infusions of 2 formulations of antineoplastons, A10 and AS2-1, and was administered for an average of 20 months. Complete responses were accomplished in 23% of patients, partial response in 8%, stable disease in 31%, and progressive disease in 38% of cases. Six patients (46%) survived more than 5 years from initiation of antineoplastons; 5 of these patients were not treated earlier with radiation therapy or chemotherapy; this may be a direct portrayal of the damaging effects chemotherapy and radiation have on the body, some chemotherapies are even known to cause cancer thus explaining the better treatment outcomes in the patients that did not undergo prior conventional treatments. The study is still ongoing and accruing additional patients. The percentage of patients’ responses is lower than that for standard treatment of favorable PNET’s, but long-term survival in low-risk cases and reduced toxicity makes antineoplaston therapy promising for very young children, patients at high risk of complications due to standard therapy, and patients with recurrent tumors (Burzynski S.R et al, June 2005, pg168-177).
To watch the first movie on this topic click here (worth the watch):

REFERENCES
Burzynski S.R; Conde A.B; Peters A; Saling B; Ellithorpe R; Daugherty J.P; Nacht C.H, “A Retrospective Study of Antineoplastons A10 and AS2-1 in Primary Brain Tumours”, Clinical Drug Investigation, Volume 18, Number 1, July 1999 , pp. 1-10(10).
Burzynski S.R; Weaver R.A; Janicki T.J, Syzmkowski B; Jurida G; Khan M; Dolgopolov V, “Long-term Survival of High-Risk Pediatric Patients With Primitive Neuroectodermal Tumors Treated With Antineoplastons A10 and AS2-1”, Integrative Cancer Therapy, June 2005 4: 168-177, doi:10.1177/1534735405276835.
Burzynski S.R; Weaver R.A; Janicki T.J; Burzynski B, “Targeted Therapy With Antineoplastons A10 and AS2-1 of High-Grade, Recurrent, and Progressive Brainstem Glioma”, Integrative Cancer Therapy, March 2006 5: 40-47, doi:10.1177/1534735405285380.
Burzynski S.R; Weaver R.A; Janicki T.J; Burzynski B, “The present state of antineoplaston research”, Integrative Cancer Therapy, 2004 March; 3(1):47-58.
Hudgins W.R; Shack S; Myers CE; Samid D, “Cytostatic activity of phenylacetate and derivatives against tumor cells”, Biochemical Pharmacology. Vol. 50, NO. 8, pp. 127Sl279, 1995.
Li X; Wang J; Li J; Wu J; Li Y; Zhu H; Fan R; Xu W, “Novel aminopeptidase N inhibitors derived from antineoplaston AS2–5 (Part I)”, Bioorganic & Medicinal Chemistry 17 (2009) 3053–3060.
Websites used for introductory information:
Hi. First, I really like your post, it's VERY informative, even if I think it's a little too long and "scientific" to be readable by non-cancer-research people. More like a real scientific review^^
You also quote your sources etc., really nice.
You should - however - increase your visibility by:
a) using the "steemstem" tag, so that the scientific steem community can find your article easier
b) writing a good introduction post about yourself and using the "introduceyourself" tag there - this will give you an initial base of followers
and yes: @OriginalWorks
Very comprehensive, informative and well researched!
Congrats on your first post!
The @OriginalWorks bot has determined this post by @pharmacy-jinni to be original material and upvoted it!
To call @OriginalWorks, simply reply to any post with @originalworks or !originalworks in your message!
Thank you so much :) I have really tried to simplify it already :D
I will definitely take your advice!
Much love
Dr. Burzynski offers free clinic tours to his facility with videos explaining his treatment methods with Antineoplastons.
I recommend EVERYONE to visit his clinic in Houston, Texas. Your visit can save a life one day by sharing his treatment method about Antineoplastons.
He also needs your support for fighting bogus lawsuits against him targeted by FDA and the fake medical establisments of USA.
Hey @gardenofeden what do you think of this alternative therapy?
ahh.....a young pharmacy student believing more than what he studies or taught!
Let's see your future articles, my friend!
Following you now!
I have seen many videos of Dr. Burzynski but not this one. I just finished the video.
The FDA & the medical institute of America are ALL corrupt. The FDA needs to be abolished along with the Natinal Cancer Institute of America.