MSG Appears To Be Safe for Many, But Not All
I was watching a Stanford video by a graduate student of Prof. Robert Sapolsky that talked about the role of
glutamate in the functioning of the brain. Because of it's role in the transference of information from neuron to neuron, I had some small hope that perhaps it might play a positive role in the treatment of dementia and other memory-related issues.


MSG is one of the most commonly-used food additives worldwide. It is ubiquitous in many countries' restaurants, and even in homes. No wonder it's so popular - it's taste, which is called "umami" aka "savory" (the "5th taste"), is very nice - sweet and salty. In color it looks like salt but its appearance is like long crystals, instead of little grains. Brands include Aji-No-Moto (the company that originally made it), Accent (arguably the most famous brand in the US), Super-Moto, Vewon, Golden Crown, Ajinotakara, Tone's, Infusions, Fufeng, Golden Pak, Jeecon, FeedStimulants, Triveni Chemicals, and many others sell it, and many famous brands from the top snack companies, including Pepsi and Coke, also use it: Doritos, Pringles, Cheese Nips, Cup Noodles, Campbells, Hamburger Helper, Maruchan (and many other instant noodle brands), and many others do or did use MSG. It is also commonly used in bouillon powder and cubes. In Indonesia, it's so popular that it's hard to find processed, restaurant, fast and junk food that is NOT made with MSG.
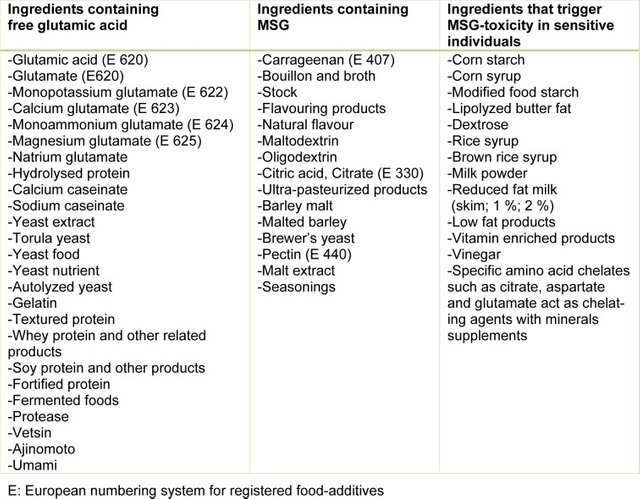
"...the average daily intake of MSG is estimated to be 0.3–1.0 g (Geha et al. 2000).", "The normal content of added MSG in food is 0.2–0.8 % for appropriate taste. Large amounts cause people to perceive the flavor unfavorably;...", "...the largest palatable dose for humans is ∼60 mg/kg body weight with higher doses causing nausea;..."
Possible Risks
Unfortunately, over the past few decades multiple studies, most of which were on animals, have demonstrated that MSG causes functional, behavioral and learning problems, including long-term memory impairment, hippocampal and cortical Na+, K+-ATPase inhibition, dyslipidemia (an abnormal amount of lipids in the blood), excitotoxicity (nerve cells suffer damage or death when the levels of otherwise necessary and safe neurotransmitters such as ***glutamate***...become dangerously high, resulting in excessive stimulation of nerve receptors), anxiety, headaches, brain damage, and other side effects. When combined with trans fats and saturated fats, the effects on memory are even more pronounced, especially in females. Although some studies report that small amounts (10mg/kg) of MSG can aid in memory retention and, thus, learning, habitual usage and larger doses have the opposite effect. Another study did not find memory improvement at even lower levels of .5-1.5 mg/kg of bodyweight, and it found mild memory dysfunction as well as anxiety.
It is important to note that MSG consumed by itself instead of within food tends to have different effects, with carbohydrates having the greatest mediating effect on MSG.
Mitigation of the Negative Effects of MSG
One study showed that selenofuranoside reduced the effects of trans fats and excessive glutamate on the hippocampus and cortex. Others showed that Allium Sativum (garlic) powder prevented MSG-induced neurotoxicity and improved short-term memory, among other positive effects. Other studies have shown that L-theanine (found in green and black tea, and in Bay Bolete mushrooms); curcumin (found in turmeric); resveratrol (found in skin of red grapes, blueberries, raspberries, mulberries, peanuts and red grape wine); DHA omega fatty acid (found in oily fish like salmon, herring, white, tuna, anchovies and mackerel; cod liver oil; egg yolks; chia, flax and hemp seeds); natto [a type of fermented soybeans]; and walnuts; magnesium (found in green leafy vegetables, such as spinach, legumes, nuts, seeds, and whole grains); and ashwaganda (found in the Indian herb Withania somnifera of the same name and has been used for centuries in Ayurvedic medicine) help as well. Black (fermented) garlic extract seemed to negate memory impairment in a tiny study. Other research also points to Vitamin C, vitamin E, quercetin and diltiazem.
Some studies suggest that sufferers of Gulf War Illness (GWI), fibromyalgia (FM) and irritable bowel syndrome (IBS) may experience relief/reduction of symptoms if they eliminate excitotoxins like MSG and aspartame from their diet.
The Blood-Brain Barrier, Unborn and Neonatal Babies
The blood-brain barrier (BBB) is a lining inside the brain's blood vessels that restricts what can be passed from the blood into the brain. This is more than just a selective osmosis: the BBB actively prevents some substances from access to the brain, although it is weak at a small number of points. Studies have shown that neonatal (within the first 28 days after birth) exposure to MSG results in an increase in glutamate in the brain because the BBB isn't fully formed yet. This suggests that such babies (<12 weeks) should not be given MSG in their food, although some meta-studies by governing bodies and scientific groups have concluded that babies are as able as adults to handle MSG. "The JECFA also noted the evidence that it was not necessary to treat pregnant women and infants as special cases; however, they did retain the previously expressed position that food additives, in general, should not be used in infant foods to be consumed before 12 wk of age." Similarly, the placental barrier acts to minimize transference of glutamate from the mother to the fetus/embryo, so pregnant women need not worry about their unborn babies.
Areas that Aren't Protected by the BBB
["Several areas of the human brain are not protected by the BBB. These include the circumventricular organs such as the area postrema, median eminence of the hypothalamus, pineal gland, and the posterior pituitary. The posterior pituitary and pineal gland are not covered by the BBB because they secrete hormones into circulation. The median eminence is not covered by BBB because the pituitary secretions collect in this area before release into circulation. The area postrema detects noxious substances present in the blood and is therefore not covered by the BBB."] (https://courses.lumenlearning.com/boundless-ap/chapter/protection-of-the-brain/) Further info here.
MSG and Memory
Some studies indicate a positive correlation between MSG and improved memory at low doses; however, the repeated use of MSG causes anxiety at all tested doses in rodents, which have been shown to be amongst the most sensitive to MSG, especially if not as part of their food. Some human studies have suggested a positive effect on demented patients, but they are subject to criticism due to unpublished results and/or conflicts of interest.
Recommendations
Injection directly into the brain: Just DON'T!
Feeding via intubation into the intestinal tract: Not recommended.
Large amounts on a daily basis: Avoid.
Pregnant women: No risk to fetuses and embryos.
Babies under 12 weeks: Potentially no risk, but recommended to minimize/avoid.
Children & adults: In moderation, it should be fine for the majority of humans but a small portion of the population may be sensitive to it, especially if consumed alone.
Memory Care Patients: More data is needed.
Sufferers of psychiatric disorders: Try eliminating excitatory neurotransmitter like MSG from your diet.
Sufferers of headaches, migraines, and pain-inducing diseases like fibromyalgia, IBS and Gulf War Illness (GWI): Eliminate MSG and other things (e.g. , caffeine, aspartame, cocoa) from your diet to see if your symptoms improve.
If you are experiencing medical problems, you can eliminate things from your diet for a few weeks or months see if anything changes. However, doing only one thing at a time may not help when there are multiple substances (separately or when combined) that are causing your condition. Consult a medical specialist (immunologist, allergist, etc.) to try and determine what substances you should try stopping. If you eat a lot of processed foods, you may find this to be challenging since things like milk and other dairy products, eggs, soy and/or wheat are present in the vast majority of prepared foods in many countries; it's advisable to learn to cook simple meals from scratch.
References
Please note that animal testing is not a reliable determination of how something will affect humans as they have different sensitivities. For example, we love chocolate and coffee many other caffeinated products, but the caffeine in them is toxic to cats and dogs, and chocolate contains the toxic (for them) compound theobromine. Within humans, there is also a great deal of variation; some humans become energized by caffeine, while others become sleepy, and some may experience heart problems. Thus, any results from animal testing should not be of much value, although testing of primates most similar to us would be more valuable. In fact, subcutaneous (below the skin) injections of MSG do not typify how animals and humans consume MSG, or reproduce the chemical reactions between MSG and the food it's in and, thus, administration of pure MSG through any type of injection - and even orally - makes the research fairly useless. Combining these factors in animal studies really means that the majority of studies that weren't performed on humans have little to no value.
MSG Injected into the Brain
Testing using intraperitoneal - or other brain - injections, thus bypassing the BBB, is not relevant except to humans with a damaged BBB. I have crossed out such sources and studies.
Wikipedia
If you go to Wikipedia, you will note that the Safety section does NOT mention any of this. Instead, use scientific resources like Google Scholar, or search the various scientific journals to find valid research results!
Editorial Inserts
I have inserted information into sources only when necessary, and indicated by [] or {}. All emphasis is by me to help show results.
Research and Metastudies (Meta-analyses), with Results and Comments
- [2021]"Monosodium glutamate (Compound)", PubChem (National Library of Medicine) excerpts:
Section 8.3: Drug Warnings
- "The large doses of sodium glutamate required for the treatment of hepatic encephalopathy may result in dangerous alkalosis and hypokalemia ... important to keep close control on the electrolyte balance during therapy." Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 59
- "Injections of sodium glutamate should be given with caution to patients with hepatic cirrhosis, impaired renal function, or liver disease not associated with hyperammonemia." Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 59
- "Food and Environmental Agents: Effect on Breast-Feeding: Monosodium glutamate: None. /from Table 7/" Report of the American Academy of Pediatrics Committee on Drugs in Pediatrics 93 (1): 142 (1994)
Section 10.2: Absorption, Distribution and Excretion
- "Glutamate is absorbed from the gut by an active transport system specific for amino acids...As a consequence of the rapid metabolism of glutamate in intestinal mucosal cells and in the liver, systemic plasma levels [of glutamate] are low, even after ingestion of large amounts of dietary protein." WHO Food Additive Series 22; L-Glutamic Acid and its Ammonium, Calcium, Monosodium and Potassium Salts. [Available as of March 20, 2007].(http://www.inchem.org/documents/jecfa/jecmono/v22je12.htm)
- "... Intestinal and hepatic metabolism results in elevation of levels in systemic circulation only after extremely high doses given by gavage (>30mg/kg body weight). Ingestion of monosodium glutamate (MSG) was not associated with elevated levels in maternal milk, and glutamate did not readily pass the placental barrier. Human infants metabolized glutamate similarly to adults." PMID:10736380, Walker R and Lupien JR; J Nutr 130 (4S Suppl): 1049S-52S (2000)
Note: "gavage" is force-feeding by supplying a nutritional substance by means of a small plastic feeding tube passed through the nose or mouth into the stomach. - "Oral administration of pharmacologically high doses of glutamate results in elevated plasma levels. The peak plasma glutamate levels are both dose and concentration dependent ... . When the same dose (1 g/kg b.w.) of monosodium glutamate (MSG) was administered by gavage in aqueous solution to neonatal rats, increasing the concentration from 2% to 10% caused a five-fold increase in the plasma area under curve; similar results were observed in mice ... . Conversely, when MSG (1.5 g/kg b.w.) was administered to 43-day-old mice by gavage at varying concentrations of 2 to 20% w/v, no correlation could be established between plasma levels and concentration ..." WHO Food Additive Series 22; L-Glutamic Acid and its Ammonium, Calcium, Monosodium and Potassium Salts. Available as of March 20, 2007.
- "Administration of a standard dose of 1 g/kg b.w. MSG by gavage as a 10% w/v solution resulted in a marked increase of plasma glutamate in all species studied. Peak plasma glutamate levels were lowest in adult monkeys (6 times fasting levels) and highest in mice (12-35 times fasting levels). Age-related differences between neonates and adults were observed; in mice and rats, peak plasma levels and area under curve were higher in infants than in adults while in guinea pigs the converse was observed." WHO Food Additive Series 22; L-Glutamic Acid and its Ammonium, Calcium, Monosodium and Potassium Salts. Available as of March 20, 2007.
Section 11.2: Uses
- "Medically it has been used to reduce blood ammonia levels in ammoniacal azotemia, therapy of hepatic coma, in psychosis, and mental retardation." National Library of Medicine - Medical Subject Headings (2007)
- "Medication: Has been used in the treatment of hyperammonemia in conditions such as hepatic encephalopathy." Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 59
Section 13.1.2: Health Hazards
- "SYMPTOMS: Large oral doses in humans have provoked burning sensation, facial pressure, chest pains, dyspnea, somnolence, hallucinations, distorted perceptions, nause [sic] and vomiting. Susceptible individuals may experience an allergic response. ACUTE/CHRONIC HAZARDS: This compound emits toxic fumes when heated to decomposition. (NTP, 1992)" National Toxicology Program, Institute of Environmental Health Sciences, National Institutes of Health (NTP). 1992. National Toxicology Program Chemical Repository Database. Research Triangle Park, North Carolina.
Section 14.1: Toxicological Information
Section 14.1.1: Acute Effects
- "L-Glutamic acid and its ammonium, calcium, monosodium and potassium salts were evaluated by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 1988. The Committee noted that intestinal and hepatic metabolism results in elevation of levels in systemic circulation only after extremely high doses given by gavage (>30mg/kg body weight). Ingestion of monosodium glutamate (MSG) was not associated with elevated levels in maternal milk, and glutamate did not readily pass the placental barrier. Human infants metabolized glutamate similarly to adults. Conventional toxicity studies using dietary administration of MSG in several species did not reveal any specific toxic or carcinogenic effects nor were there any adverse outcomes in reproduction and teratology studies. Attention was paid to central nervous system lesions produced in several species after parenteral administration of MSG or as a consequence of very high doses by gavage. Comparative studies indicated that the neonatal mouse was most sensitive to neuronal injury; older animals and other species (including primates) were less so. Blood levels of glutamate associated with lesions of the hypothalamus in the neonatal mouse were not approached in humans even after bolus doses of 10 g MSG in drinking water. Because human studies failed to confirm an involvement of MSG in "Chinese Restaurant Syndrome" or other idiosyncratic intolerance, the JECFA allocated an "acceptable daily intake (ADI) not specified" to glutamic acid and its salts. No additional risk to infants was indicated. The Scientific Committee for Food (SCF) of the European Commission reached a similar evaluation in 1991. The conclusions of a subsequent review by the Federation of American Societies for Experimental Biology (FASEB) and the Federal Drug Administration (FDA) did not discount the existence of a sensitive subpopulation but otherwise concurred with the safety evaluation of JECFA and the SCF." PMID:10736380,
Walker R and Lupien JR; J Nutr 130 (4S Suppl): 1049S-52S (2000)Section 14.1.5: Human Toxicity Excerpts
- "/HUMAN EXPOSURE STUDIES/ ... A high dose of 2.5 g was tested in 6 healthy controls and 30 asthmatics (7: allergic asthma; 15: intrinsic asthma with intolerance to aspirin; 8: intrinsic asthma with aspirin intolerance, intolerance to alcohol or to food additives). Two patients presented with a mild bronchospasm, occurring 6 to 10 hours after the ingestion. Different mechanisms are discussed. A cholinergic mechanism might be incriminated, either due to stimulation of the synthesis of acetylcholine, or due to a vagal reflex elicited by a reflux esophagitis. However, a high vagal hyperreactivity seems to be needed for the occurrence of asthma. It is concluded that a very small subset of patients with intrinsic asthma might present with an intolerance to monosodium glutamate if high doses are consumed." Moneret - Vautrin DA; Allerg Immunol (Paris) 19 (1): 29-35 (1987)
- "/HUMAN EXPOSURE STUDIES/ Monosodium glutamate is widely regarded as the provocative agent in the "Chinese restaurant syndrome," of which flushing is regarded as part of the reaction. Six subjects were monitored by laser Doppler velocimetry for changes in facial cutaneous blood flow during challenge with monosodium glutamate and its cyclization product, pyroglutamate. Additionally, records of patients challenged with monosodium glutamate in the laboratory were reviewed. No flushing was provoked among the twenty four people tested, eighteen of whom gave a positive history of Chinese restaurant syndrome flushing. These results indicate that [MSG-]provoked flushing, if it exists at all, must be rare. Monosodium glutamate and its cyclization product, pyroglutamate, may provoke edema and associated symptoms." PMID:3745527, Wilkin JK; J Am Acad Dermatol 15 (2): 225-30 (1986)
- "/HUMAN EXPOSURE STUDIES/ /The objective of this study was/ to determine whether monosodium glutamate (MSG) would induce bronchoconstriction in a group of adults with asthma who perceived that they were MSG sensitive. Twelve subjects (seven women, mean age 35.3 years) with clinically documented asthma and a perception of MSG-induced asthma were recruited. FEV1 and peak expiratory flow data were obtained for 3 whole control days, as well as time-matched data for 3 separate challenge days (1 g MSG, 5 g MSG, and 5 g lactose [placebo]). Opaque capsule challenges were given as a single dose in the morning after an overnight fast. Subjects complied with an elimination diet throughout the study. Nonspecific bronchial hyperresponsiveness was measured at baseline, after the control days, and at the conclusion of the challenges. Venous blood samples were taken at baseline and on each challenge day to determine soluble inflammatory marker (eosinophil cationic protein and tryptase) activity. No immediate or definite late asthmatic reactions occurred. One subject's FEV1 declined more than 15% on MSG challenge, but 95% confidence limits for the control-day spirometry showed that this decline was within her daily variation. ... No significant changes in bronchial hyperresponsiveness or soluble inflammatory markers were found." PMID:9648703, Woods RK et al; J Allergy Clin Immunol 101 (6 Pt1): 762-71 (1998)
- "/HUMAN EXPOSURE STUDIES/ This study sought to determine the prevalence of reactions to additives, including monosodium glutamate (MSG), in patients with chronic urticaria using a rigorous protocol. Sixty-five subjects (44 women, 21 men; ages 14-67) /were studied/. All had urticaria for >6 wk without discernible etiology. Subjects with active urticaria were studied while they were taking the lowest effective dose of antihistamine. Screening challenges to the 11 additives most commonly associated with exacerbations of chronic idiopathic urticaria were performed in a single-blind fashion. The dose of MSG given was 2500 mg. Skin scores were obtained to determine a positive reaction in an objective manner. Subjects with a positive screening challenge were rechallenged (at least 2 wk later) with a double-blind, placebo-controlled protocol as in-patients in our General Clinical Research Center. Two subjects had positive single-blind, placebo-controlled challenges, but neither had a positive double-blind, placebo-controlled challenge. It is concluded, with 95% confidence, that MSG is an unusual (<3% at most) exacerbant of chronic idiopathic urticaria." , PMID:10736383, Simon RA; J Nutr 130 (4S Suppli): 1063S-6S (2000)
Section 14.1.6: Non-Human Toxicity Excerpts
- "/LABORATORY ANIMALS: Acute Exposure/ Monosodium glutamate was not sensitizing in the guinea pig maximization test. /from table/", European Chemicals Bureau; IUCLID Dataset, Sodium Hydrogen Glutamate (CAS No.142-47-2). Available as of January 22, 2007
- "/LABORATORY ANIMALS: Acute Exposure/ Monosodium glutamate was described as not irritating in the rabbit test according to the EEC method described in the annex to directive 92/69/EEC, part B, Method B4." European Chemicals Bureau; IUCLID Dataset, Sodium Hydrogen Glutamate (CAS No.142-47-2). Available as of January 22, 2007
- "/LABORATORY ANIMALS: Acute Exposure/ Groups of 10-12-day old Swiss Webster albino mice, each containing 7-23 animals, were given single oral doses of MSG at levels of 0.25, 0.50, 0.75, 1.0, or 2.0 g/kg. Groups of 2 or 4 mice of the same age were given single oral doses of either 1.0 or 3.0 g/kg L-glutamic acid or monosodium-L-aspartate or 3.0 g/kg L-glutamate-L-aspartate, monosodium glutamate, NaCl, L-glycine, L-serine, L-alanine, L-leucine, D,L-methionine, L-phenylalanine, L-proline, L-lysine, L-arginine, or L-cysteine. The animals were sacrificed after dosing and brains were examined by either light or electron microscopy. The severity of brain damage was estimated by quantifying the pathological changes in the hypothalamus. One g/kg of glutamic acid destroyed approximately the same number of hypothalamic neurons as a comparable dose of MSG. Of the amino acids tested, only aspartate and cysteine produced hypothalamic damage. These amino acids caused both retinal and hypothalamic lesions similar to those found after treatment with MSG." WHO Food Additive Series 22; L-Glutamic Acid and its Ammonium, Calcium, Monosodium and Potassium Salts. Available as of March 20, 2007
- "/LABORATORY ANIMALS: Acute Exposure/ At birth, the rat brain glutamate concentration is about 4 mM and increases over a period of 20 days to the adult value of approximately 10 mM. When a 4 g/kg dose was given intragastrically, convulsions were seldom observed, and then only after 90 minutes. [That dosage is VERY large.]" WHO Food Additive Series 22; L-Glutamic Acid and its Ammonium, Calcium, Monosodium and Potassium Salts. Available of March 20, 2007
Hazardous Substances Data Bank (HSDB)
Note: In Sections 10.2 and 14.1.3, some studies define "an extremely high dose" as "(>30mg/kg body weight)." Others in 10.2 consider 1 g/kg BW to be "high". Without a standard definition, of what is "high", let alone "extremely high", it is hard to know what is excessive.
[2016 ]Effect of monosodium glutamate on cognitive function in people with dementia{200-person, University hospital Medical Information Network (UMIN) Clinical Trial Registration (CTR), Japan, that was sponsored by Tottori University, Japan, and paid for by Ajinomoto Company, Inc. The results were NOT published, which is suggestive. Because of conflicts of interest and no access to the full data, this study is subject to criticism.}[2019]"Effect of monosodium L-glutamate (umami substance) on cognitive function in people with dementia", , "Conflict of interest statement: Katsuya Urakami owns a patent on the Touch Panel-type Dementia Assessment Scale and receives royalties from Nihon Kohden Corporation. Hideki Matsumoto is employed by Ajinomoto Co., Inc. The remaining authors declare that they have no conflict of interest". Results: "At the follow-up assessment {after MSG intake was stopped}, the TDAS total scores in the MSG group showed significant improvement compared with those reported in the Control group (p < 0.05). Furthermore, there was a correlation of changes from pre-intervention to post-intervention between the TDAS and enjoyment of the meal (r = -0.299, p = 0.049)." Conclusions: "Our results suggest that continued ingestion of MSG has an effect on cognitive function." Because of conflicts of interest, this study is subject to criticism.- [
[2018]Oral administration of] Selenofuranoside improves long-term memory deficits in rats after exposure to monosodium glutamate: Involvement of Na+, K+-ATPase activityOral administration of MSG (2 g/kg) and/or selenofuranoside (5 mg/kg) to only 24 non-neonatal (starting at 5 weeks) rats for 10 days. "Results: The results demonstrate that the administration of MSG led to long-term memory impairment, as shown in the SDPA task, and also hippocampal and cortical Na+, K+-ATPase inhibition. There were no alterations in the AChE activity and oxidative stress parameters. Treatment with selenofuranoside attenuated memory impairment associated with MSG exposure by improving the hippocampal Na+, K+-ATPase activity." I do not consider this to be an important study due to the small number of subjects and the excessive amount of MSG used. - [2010]Dietary trans-fat combined with monosodium glutamate induces dyslipidemia and impairs spatial memory "Dietary trans-fat combined with MSG increased central adiposity, promoted dyslipidemia and impaired spatial learning." Initial age of rats, duration of trial, and method of administration of MSG and trans fat are not shown in the abstract, and it's behind a pay wall.
- 2020 non-scientific video 6 Supplements to Combat Glutamate Excitotoxicity
[2020]Neuroprotective Potential of Allium sativum against Monosodium Glutamate-Induced Excitotoxicity: Impact on Short-Term Memory, Gliosis, and Oxidative StressInvalid study due to injection of both substances into the brain, thus bypassing the BBB. Also, I believe that the variance in administration of substances introduced too many variables that clouded the results. 40 rats that weren't neonatal (starting at one month in age) were used for 30 days using low doses.- Changes in Spontaneous Working-memory, Memory-recall and Approach-avoidance following “Low Dose” Monosodium Glutamate in Mice "MSG administration was associated with significant anxiolytic and memory-enhancing effects at 10 mg/kg (after both acute and repeated dosing); however, higher doses used in this study were associated with significant anxiogenesis and memory retardation. Hippocampal glutamate and glutamine levels did not increase significantly at any of the doses of MSG. In conclusion, MSG administration at low doses was associated with significant changes in hippocampal-dependent behaviours without a concomitant significant shift in hippocampal glutamate/glutamine levels." 180 adult mice were tested, oral gavage administration of 10, 20, 40 or 80 mg/kg BW over 3 weeks. I.E" At 10 mg/kg, it improved memory (spatial-recognition and spatial working-memory) and reduced anxiety, and at 20 mg/kg it reduced anxiety only, but at the other doses the opposite happened; however, repeated administration of MSG caused anxiety at all doses. This study also notes that other studies that used very low doses (.5-1.5 mg/kg) showed no effect, and those that used doses >=40 mg/kg also caused nerve damage as well as memory issues and anxiety. It goes on to point out that studies which used amounts in excess of normal human consumption levels, and/or if injected or delivered by nasogastric intubation (a tube from the nose to the intestinal tract), were questionable because they bypassed the nerves in the mouth and gut.
[2000]Glutamate and aspartate impair memory retention and damage hypothalamic neurons in adult miceDetails not available because it's behind a paywall, but the low dosage of MSG (4.0 mg/g) was injected into the brains of mice, so this is invalid.- [2012]Elevated Plus Maze and Y-Maze Behavioral Effects of
Subchronic, Oral Low Dose Monosodium Glutamate in Swiss
Albino Mice "The study concluded that subchronic MSG at the doses administered was anxiogenic but had only a
slight retardant effect on spatial working memory. " 20 of 40 adult mice were given 0.5, 1.0 and 1.5 mg/kg doses of MSG by intubation. The size of this study and method of dosing make its value poor. - [2017]The effects of black garlic on the working memory and pyramidal cell number of medial prefrontal cortex of rats exposed to monosodium glutamate "There were no significant differences between groups in the estimated total number of pyramidal cell of medial prefrontal cortex. The MSG may not cause the death of neurons, but it may modify neuronal architectures that are sufficient to disrupt memory functions. Black garlic may play a role as an antioxidant agent that prevents the prefrontal cortex from glutamate-induced oxidative stress. It is concluded that the ethanolic fermented garlic extract prevented the working memory impairment following MSG administration." Only 25 adolescent rats were used in 5 groups, 2 of which were controls, were given MSG and garlic extract (0.0125, 0.025, and 0.05 mg/g bw, respectively) over 10 days, so this study is too small and short to be conclusive.
- 2016 Metastudy of human trials: Does monosodium glutamate really cause headache? : a systematic review of human studies "Because of the absence of proper blinding, and the inconsistency of the findings, we conclude that further studies are required to evaluate whether or not a causal relationship exists between MSG ingestion and headache." The researchers looked at 10 studies, 5 of which were with food and the dose of MSG was 1.5 – 3.15 g, and the other 5 were without food and the dose of MSG was 1.25 – 12 g. MSG and placebos were given in capsules, water, beverages or food. Out of all the studies reviewed, the number of participants varied from 11 to 130; with food: 24-73; without food (water, broth or beverage): 11-130. Most studies found no evidence of headaches, but some found them, especially among females. Some studies showed male and female while others had only one gender; most didn't mention race.
- 2018 Metastudy of human and animal trials: Extensive use of monosodium glutamate: A threat to public health? "In conclusion we would like to state that although MSG has proven its value as an enhancer of flavour, different studies have hinted at possible toxic effects related to this popular food-additive. These toxic effects include CNS disorder, obesity, disruptions in adipose tissue physiology, hepatic damage, CRS and reproductive malfunctions. These threats might have hitherto been underestimated...Further studies need to be undertaken in order to assess the connection between MSG and cardiovascular disorders, headache, and hypertension in human models."
- 2000 Metastudy of human and animal trials: The Safety Evaluation of Monosodium Glutamate
"In its conclusion on this matter, the JECFA stated “controlled double-blind crossover trials have failed to demonstrate an unequivocal relationship between ‘Chinese Restaurant Syndrome’ and consumption of MSG. MSG has not been shown to provoke bronchoconstriction in asthmatics.”," "Because human studies failed to confirm an involvement of MSG in “Chinese Restaurant Syndrome” or other idiosyncratic intolerance, the JECFA allocated an “acceptable daily intake (ADI) not specified” to glutamic acid and its salts. No additional risk to infants was indicated. The Scientific Committee for Food (SCF) of the European Commission reached a similar evaluation in 1991. The conclusions of a subsequent review by the Federation of American Societies for Experimental Biology (FASEB) and the Federal Drug Administration (FDA) did not discount the existence of a sensitive subpopulation but otherwise concurred with the safety evaluation of JECFA and the SCF."
"The FASEB report concludes that there is no evidence to support a role for dietary MSG or other forms of free glutamate in causing or exacerbating serious, long-term medical problems resulting from degenerative nerve cell damage. The FDA accepted the conclusion that serious neurotoxicologic effects from MSG are limited to animals given very large doses by parenteral, pharmacologic or other nondietary conditions of use or administration."
"With regard to the potential disruption of the neuroendocrine axis, the FASEB Expert Panel gave particular consideration to the potential of dietary MSG to affect adversely the structure and function of areas of the brain not protected by the blood-brain barrier...The FDA concurred with the conclusion (from animal studies) that large doses of glutamate can influence hormonal function but concluded further that it did not believe that there was evidence to indicate that MSG as ordinarily consumed in foods disrupts the neuroendocrine axis in humans."
"It has been contended in some quarters that glutamate in commercial products such as MSG or hydrolyzed protein, is different in some way from naturally occurring glutamate. The FASEB Panel rejected this contention.
Finally, the FDA interpreted the findings of the FASEB Report to be generally consistent with the safety assessments of other authoritative organizations (presumably including the JECFA and SCF) that have affirmed the safety of MSG at levels normally consumed by the general population, and concurred with the conclusion that there is no evidence linking current MSG food use to any serious, long-term medical problems in the general population." - 2014 Metastudy of human and animal trials: Is there a relationship between dietary MSG obesity in animals or humans?
"Hypotheses positing dietary MSG effects on body weight involve results from rodent MSG injection studies that link MSG to actions in brain not applicable to MSG ingestion studies. The fundamental reason is that glutamate is metabolically compartmentalized in the body, and generally does not passively cross biologic membranes. Hence, almost no ingested glutamate/MSG passes from gut into blood, and essentially none transits placenta from maternal to fetal circulation, or crosses the blood–brain barrier. Dietary MSG, therefore, does not gain access to brain. Overall, it appears that normal dietary MSG use is unlikely to influence energy intake, body weight or fat metabolism." - 2013 Metastudy of human and animal trials: Monosodium Glutamate Toxic Effects and Their
Implications for Human Intake: A Review "Animal studies in which MSG was administered perorally in doses similar to average human intake or intake of extreme users showed that MSG led to disturbances in metabolism with the increase in more parameters including insulin, fatty acids and
triglycerides in serum, MSG increased the expression of several genes implicated in adipocytes differentiation, it affected the liver function resulting in elevation of transaminaseslevels and bile synthesis, it also led to oxidative stress in liver and to the pathological changes in ovaries and fallopian tube. MSG intake in human studies was associated with increased levels of several circulating amino acids, however no changes in the postprandial glucose and insulin were found, which was in contradiction to animal studiesresults.
The relationship between MSG and increased hemoglobin was shown in men and MSG intake was associated with
headache and subjectively reported pericranial muscle tenderness. Chinese restaurant syndrome and asthma were not
proved to be associated with MSG intake. Vitamin C, vitamin E, quercetin and diltiazem had protective effects on MSG-induced toxic changes. " This study rightly points out the differences between human and animal consumption and effects, and they were quite apt to state that research using corresponding dosages for animals should be based on normal human intake, as well as several other points that require further research.
"Thus, further animal studies focused on MSG effects on the CNS [central nervous system] in diet should equalize the doses to human intake. Further research should be done to elucidate the behavioral effects of peroral intake of MSG in various doses and potentially different influence of MSG on fetal, neonatal and adult brain structures in humans. " - [1979]Placebo-controlled studies of human reaction to oral monosodium L-glutamate "It is now possible to say with confidence that with concentrations of MSG of the order of 0.75%, it is extremely unlikely that any of the symptoms will be experienced by even a demonstrably sensitive individual. Furthermore, at a level of 1.5%, only a few individuals will be affected."
- [2001 human case study]Relief of fibromyalgia symptoms following discontinuation of dietary excitotoxins [MSG & Aspartame];J D Smith, C M Terpening, S O Schmidt, J G Gums; PMID: 11408989 DOI: 10.1345/aph.10254 "Case summary: Four patients diagnosed with fibromyalgia syndrome for two to 17 years are described. All had undergone multiple treatment modalities with limited success. All had complete, or nearly complete, resolution of their symptoms within months after eliminating monosodium glutamate (MSG) or MSG plus aspartame from their diet. All patients were women with multiple comorbidities prior to elimination of MSG. All have had recurrence of symptoms whenever MSG is ingested."
- [2012 human study]The effect of dietary glutamate on fibromyalgia and irritable bowel symptoms; Kathleen F Holton, Douglas L Taren, Cynthia A Thomson, Robert M Bennett, Kim D Jones; PMID: 22766026 "Results: The MSG challenge, as compared to placebo, resulted in a significant return of symptoms (total symptom score, p<0.02); a worsening of fibromyalgia severity as determined by the FIQR (p<0.03); decreased quality of life in regards to IBS symptoms (IBS QOL, p<0.05); and a non-significant trend toward worsening FM pain based on visual analogue scale (VAS, p<0.07).
Conclusions: These findings suggest that dietary glutamate may be contributing to FM symptoms in some patients. Future research on the role of dietary excitotoxins in FM is warranted." - [2014 human study]Monosodium glutamate and aspartame in perceived pain in fibromyalgia; María Y Vellisca, José I Latorre; PMID: 23765203 DOI: 10.1007/s00296-013-2801-5 "The discontinuation of dietary MSG and aspartame did not improve the symptoms of fibromyalgia."
- [2020 human study]The Low Glutamate Diet Effectively Improves Pain and Other Symptoms of Gulf War Illness; Kathleen F Holton, Anna E Kirkland, Michael Baron, Shalini S Ramachandra, Mackenzie T Langan, Elizabeth T Brandley, James N Baraniuk; PMID: 32859032 PMCID: PMC7551234 DOI: 10.3390/nu12092593 "these results suggest that the low glutamate diet can effectively reduce overall symptoms, pain and fatigue in GWI, but differential results upon challenge suggest that other aspects of the diet, or underlying differences within the population, may be driving these changes. Future research is needed to identify potential nutrient effects, biomarkers, and underlying metabolic differences between responders and non-responders."
- [2016 metastudy]Influence of pro-algesic foods on chronic pain conditions; Brian Edwin Cairns; PMID: 26900907 DOI: 10.1586/14737175.2016.1157471](https://pubmed.ncbi.nlm.nih.gov/26900907/) "While all four substances [monosodium glutamate, aspartame, arachidonic acid, and caffeine] are associated with pain, decreased consumption of them does not consistently reduce pain."
- [2017 human study]Effect of exclusion of frequently consumed dietary triggers in a cohort of children with chronic primary headache; Sepideh Taheri,PMID: 28298151 DOI: 10.1177/0260106016688699](https://pubmed.ncbi.nlm.nih.gov/28298151/) "Results: One hundred patients attended follow-up. Of these 13 (13%) did not respond to dietary exclusion; 87 (87%) achieved complete resolution of headaches by exclusion of 1-3 of the identified food(s). Caffeine was the most common implicated trigger (28), followed by monosodium glutamate (25), cocoa (22), aspartame (13), cheese (13), citrus (10) and nitrites (six).
Conclusions: This study demonstrates the potential scale and significance of seven frequently consumed foods or food additives as triggers for primary headache in children. Also this is the first study to show that headaches can be triggered by the cumulative effect of a food that is frequently consumed, rather than by single time ingestion." - [2020 metastudy]Could Dietary Glutamate Play a Role in Psychiatric Distress?; A Zarina Kraal, Nicole R Arvanitis, Andrew P Jaeger, Vicki L Ellingrod; PMID: 30699435 PMCID: PMC6667320 DOI: 10.1159/000496294](https://pubmed.ncbi.nlm.nih.gov/30699435/)
[2017 case study]Chinese Restaurant Syndrome; Himmatrao Saluba Bawaskar, Pramodini Himmatrao Bawaskar, and Parag Himmatrao Bawaskar; PMID: 28197052Based on the description and what helped the patient, it seems more likely that the problem was a combination of an allergic reaction to an unknown substance and an infection. To assume it was because of MSG is presumptive and not founded on empirical data.
If you appreciate this article, please 🏅upvote/like👍
 , 🤩resteem/share
, 🤩resteem/share and share it to Facebook
and share it to Facebook , Twitter
, Twitter , Reddit
, Reddit , LinkedIn
, LinkedIn and wherever else
and wherever else you can!
you can!
(non-human animals excluded; TDLo test type)
Organism Route Dose Effect/Reference women oral 50 mg/kg (50 mg/kg) LUNGS, THORAX, OR RESPIRATION: DYSPNEA, New England Journal of Medicine., 305(1154), 1981 human oral 43 mg/kg (43 mg/kg) BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY); BEHAVIORAL: HALLUCINATIONS, DISTORTED PERCEPTIONS; GASTROINTESTINAL: NAUSEA OR VOMITING, Hygiene and Sanitation, 36(9)(364), 1971 man oral 3571 ug/kg (3.5710000000000002 mg/kg) SKIN AND APPENDAGES (SKIN): DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE, Lancet., 1(988), 1987 human intravenous 714 ug/kg (0.71399999999999997 mg/kg) BEHAVIORAL: HEADACHE, Science., 163(826), 1969 [PMID:5764480] Note: "Dyspnea" means difficulty breathing, such as you might feel during an asthma attack or a sufficiently strong allergic attack.
Section 14.1.3: Toxicity Summary