How Corrosion Occurs - Basic Electrochemistry
Intro
Have you ever wondered, why metals corrode but plastic and wood do not? Take a look at household item such as pipes. If you look at your bathroom taps and if they are made out of metal, there is high chance that you will notice brown spot, which is an indication of a corrosion phenomenon. For plastic taps, they will fail or break without any corrosion.
Short Answer to Why Corrosion Occurs
The short answer is, metals can exchange electrons with each other. For plastic/polymer/wood, there is no exchange of electrons. Hence, no corrosion occurs. The exchange of electrons occur through oxidation & reduction reactions, where oxidation reaction (loss of electron) occurs at anodes while reduction reaction (gain of electron) occurs at cathodes. To better understand this, a diagram is attached for reference:
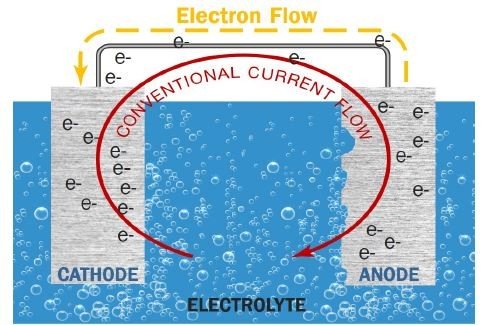
As you can see from above, the electrons,(e-) travel from anode to cathode through a metallic path. Also note that when anode loses electrons, it corrodes. Hence from this, we can say that corrosion will occur if all of these exist:
- Anode: This is where metal is lost (corroded) and electrons are given up
- Cathode: This is where electrons are consumed/gained/received
- Metallic path: Conducts electrons from anode to cathode
- Electrolyte: Allow flows of ions (i.e moisture, water)
The Famous Galvanic Series
Perhaps you may have seen or heard the galvanic series back in the school days. For a quick recap, galvanic series is a list of metals in order of their potentials (voltage) in one specific environment (i.e-seawater). The main purpose of the list is to determine the interactions between metals when they are coupled together. For instance, 2 different metals that are further apart will tend to corrode quicker. On the whole, the active metal (more -ve potential) will become the anode and corrode while the passive metal (more +ve potential) will become the cathode but corrode very slowly.
For visualization purposes, the diagram for the galvanic series in seawater is shown here for your reference:
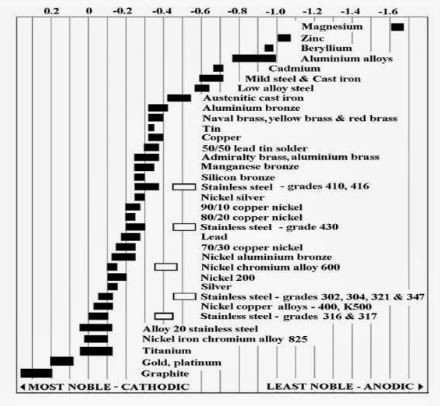
Lets do some examples.
Case 1: Joining together copper and zinc.
According to the galvanic series, the zinc is more active than copper which means, we are expecting the zinc to corrode prior to the copper.
Case 2: Coupled together stainless steel with zinc
Again, the zinc will corrode first but with a higher driving force for the electrochemical reactions. The corrosion effect will be greater (looking more bad) than case 1 since they are further apart (larger potential difference) in the galvanic series.
That's all from me now. I hope you do learn something and enjoy reading this post.
Till then, thank you.
Cheers,
Common Terminologies in Corrosion
Electron: Part of atom (subatomic particle) which has negative charge. Travels from anode(-ve) to cathode(+ve).
Ion : A charged atom or molecule. Anion is negatively(-ve) charged while cation is positively(+ve) charged.
Electrolyte: A liquid that contains ion.Can be highly conductive or mildly conductive. Higher ions in electrolyte leads to higher conductivity.
Anode: Negative electrode
Cathode: Positive electrode

Congratulations @rahim.rahman! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOP