Corrosion - Formation of Corrosion Cells
Intro
In my previous post, I have often used 2 dissimilar metals as example when describing about corrosion cell (electrochemical cells), where a complete circuit is formed when (i) : there is electrical contact between anode and cathode via a metallic path (wire) and (ii): ions can flow through the electrolyte. However, corrosion cell can also occur in a single (isolated) metal. For example, household gates or fences are often built with a single metal material (mild steel) and overtime, they will corrode although without coupled with another metal.
Corrosion Cell - Single Metal
Let us begin with comparison of diagram between the 2 corrosion cells. They are shown below:
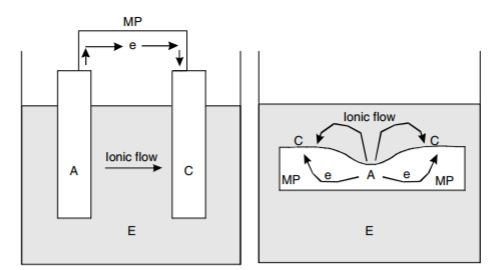
A= anode, C=cathode, E=electrolyte, e=electron, MP=metallic path
As can be seen, they are not that different. Corrosion cell occurring within a single metal can arise due to many factors. Do take note that, in reality there is no single material in this world that is perfectly uniform in characteristics. The major factors that contribute to this phenomenon can include:
- Differences in microstructure of the material (i.e- differences in grain boundary orientation, many phases within the material)
- Foreign material within the material (i.e - inclusions like oxides, sulfides which promotes anodic/cathodic site)
- Differential aeration (i.e- lower oxygen concentration promotes anodic site [such as metal traps within soil])
- Heat effect (i.e - welding job, metal heat treatments)
- Mechanical work (i.e-strained areas tend to become anodic site)
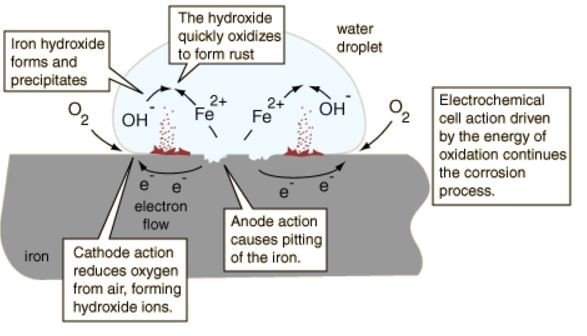
Let's take for example, a piece of iron that is left outside and exposed to moisture. Due to exposure with moisture (i.e-droplet of water), the iron surface that is in contact with water droplet becomes anode while the iron at the edge of the water droplet becomes cathode. Electron will move from the anodic site to the cathodic site within the metal itself. The reactions for this phenomenon are:
- Oxidation: Fe --> Fe(2+) + 2e(-)
- Reduction: O(2) + 2H(2)O + 4e(-) --> 4OH(-) [Just outside water droplet]
The hydroxide ions [4OH(-)] can move towards the water droplet and react with Fe(2+) ions to form iron (II) hydroxide:
- Iron (II) hydroxide: Fe(2+) + 2OH(-) --> Fe[(OH)(2)]
The iron hydroxide is then oxidized to form rust. For ease of visualisation, we may refer to the figure below:
I hope this post will allow others to understand more about corrosion phenomenon in general.
Stay tuned for more posts.
Cheers,