Acid and Base
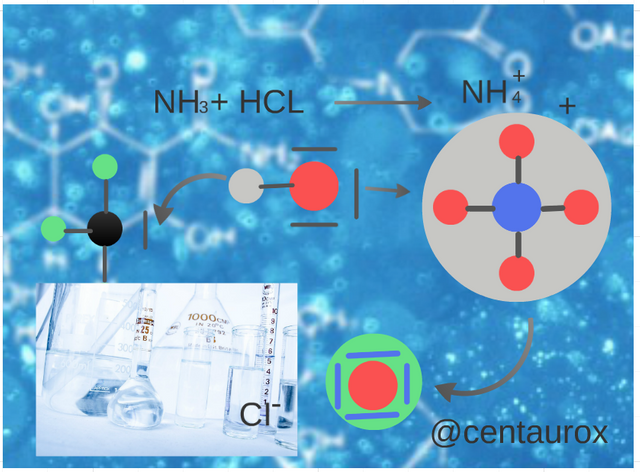
Image edited for @centaurox
The first modern definition of acids and base owes to itself the Swedish chemist Svante Arrthenius (1859 - 1927), who defined the acids as the stays that you increase the concentration of ions hidronio (H3O). Although the theory of Arrthenius, he explains the acid or basic behavior of a big number of substances, it turns out to be very limited, giving an example, he does not allow to explain the basic character of the ammonia (NH3).
A wider approach was given by the Danish chemist Johannes N. Bronsed (1879 – 1956) and the English chemist Thomas M. Lowry (1874 - 1936), those who defined an acid as as that one substances capably of transferring a proton to other substances and a base as that one substances gelds of accepting a proton of an acid, that is to say, an acid is a giver of protons and a base is a recipient of protons. The theory theory of Bronsted and Lowry, it allows to correct the problem, which was raising the behavior of the ammonia. In general, any reaction acid – base, according to the theory of Bronsted and Lowry, can be represented of the following way:
He says to himself that an acid is strong when his brought together base is weak, análogamente, a base is strong when his brought together acid is weak, the substances as the water can behave indistinctly, like acid or as base receiving in the name of anfipróticas or anfóteras. The fortitude of the acids comes give quantitatively for his constant of acidity, called also constant iónica, Ka, which it is possible to calculate for the general reaction:
Ka = [To-] [H3O] / (THERE) [IS]
In the same way, the fortitude of the base comes given by his down ones of basicidad, Kb, which it is possible to calculate:
Kb = [B] [OB] / [BOH]
The constant of acidity of an acid and the constant of basicidad of his brought together base comes related by the following expression:
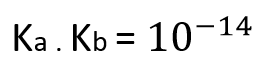
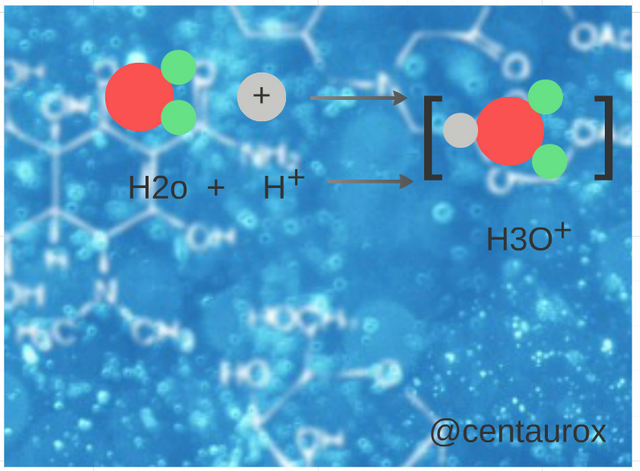
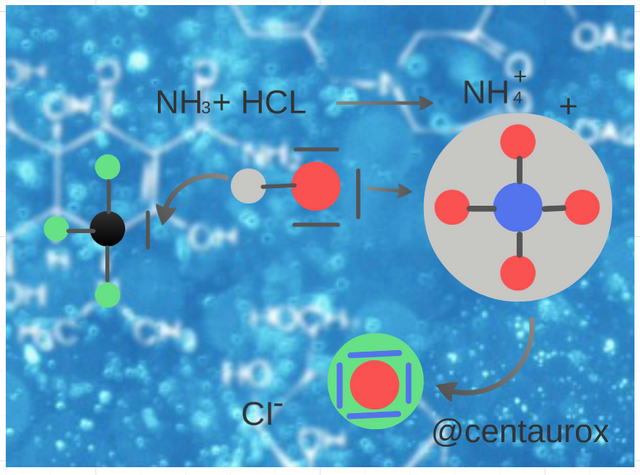
The watery ones of ammonia are basic, since the water acts as acid, transferring a proton to the molecule of NH3, which acts as a base. (Image edited for @centaurox)
Las acuosas de amoniaco son básicas, puesto que el agua actúa como ácido, cediendo un protón a la molécula de NH3, que actúa como una base. (Imagen editada por @centaurox)
Scale of pH.
When an acid or a base dissolves in water, ions H3O and OH form-, that they cause the acid or basic character of the dissolution, now well, since the pure water has a behavior anfiprótico and is autoionized according to the equation:

Indicators.
An indicator is a few substances that it changes color inside a small interval of pH, because it can exist in two or more forms, which have different structures and present different colors. The indicators are used, to measure in a brought near way the pH, of the dissolutions, since in general, the interval of turn (I change color), of the indicators is of a few 2unidades of pH, it is named a universal indicator, to a miscellany in equal volumes of dissolutions of the following indicators: red of methyl, naftolftaleína, timolftaleína, fenolftaleína and blue of bromine timol.
Acid Volumetrías – base.
The procedure used, to determine the concentration of an acid or basic dissolution is named an evaluation of dissolution and is carried out for volumetría, that is to say, measuring volumes. The evaluation of an acid dissolution receives the name of acidimetría, whereas the evaluation of a basic dissolution receives the name of alcalimetría and is in a similar way.
Acids and bases at industrial level.
It is important to knowing the following thing, since the acids and the bases are in our daily environment, in the food, the medicines, the products of cleanliness, and some others, but more to already of these chemical products, his application they help: To verify that the transformation of matter is carried out, to quantify the obtained volume, percentage of error in the process, quality control of the process, to visualize points where decrease of costs could do; that's why his application is important, company agroindustriales, agrochemical, procesadoras of food, petrochemical, for a few examples as it is the case to visualize points where there could be escapes of raw material and losses.
The discovery of the organic acids, especially carboxílicos, is narrowly related to the development of the experimental and biochemical chemistry. Since then the important events in the engineering, the biochemistry and the microbiology (the transference of oxygen, the scale and the design of means of farming, between others) have allowed the process application industrial scale.
The mathematical treatment comes down to the calculation of the concentration of the dissolution valued from the volume of dissolution valorante measured on the burette and of his concentration. Since the mathematical application in the world of the chemistry, when one works with acid and base, we are the determination of the point of equivalence it was something more difficult since the visual or graphic determination of the point of inflection of the curve has associated an important error of calculation, one resorts to the application of or the use of derivatives.
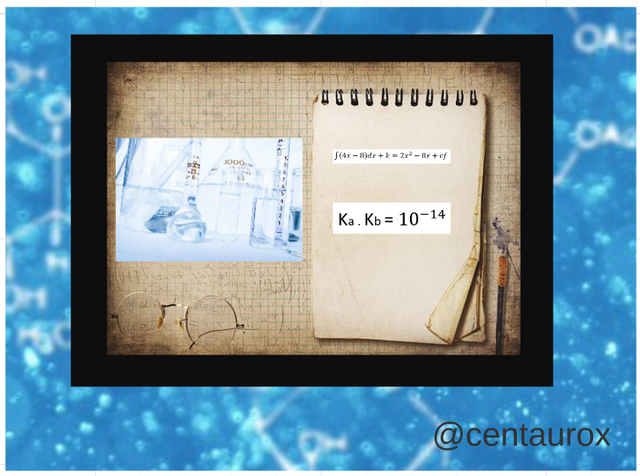
Image edited for @centaurox
In the nature we find:
Acids and bases used in the biotechnology of plants, since it is a case of the coriander and the parsley, are good determinants when you have the soils raised level of pH, owed the canister parecencia of acidity, these farmings, it puts itself of yellow color, this is for the reaction of the following way for the acidity of the ground, the molecules hidroliza spontaneously liberating ethylene, as accelerating agent of maturation, up to the case as if these farmings, the death was provoked, because this chemical reaction, it it makes to rot warm up to the point, that's why it is good to know the state of the grounds that is desirable to be cultivated and that nutrientes or base needs to level the Ph, and to have good he harvests and taken care delos farmings, this way to support the ecology of the grounds.

Image edited for @centaurox @centaurox
The purple cabbage
They can be used as warning acid - base, to obtain the pigment, it will be necessary to leave the cabbage grated in a race with water, where it will be waved occasionally. When the water is of a strong red color, it is spilt carefully, in such a way that I eliminated the most possible the cabbage.
At level of the biology, it performs supreme importance in the alive beings, since the carbonic acid is fundamental to support the pH, in the blood, also the lactic acid and the acid butanoico as the case of the milk and the butter, they form for the bacterial action on the hydrates of carbons.
The stomach inside this organ we find a fluid (gastric Juice), which is segregated by the glands of the membranes mucous, that it wraps to the stomach; in three other substances it contains hydrochloric acid, the pH of the juice costs around 1,5.
Ants are bearers of acid fómico, when it stings they liberate this acid that's why it swells up at once.
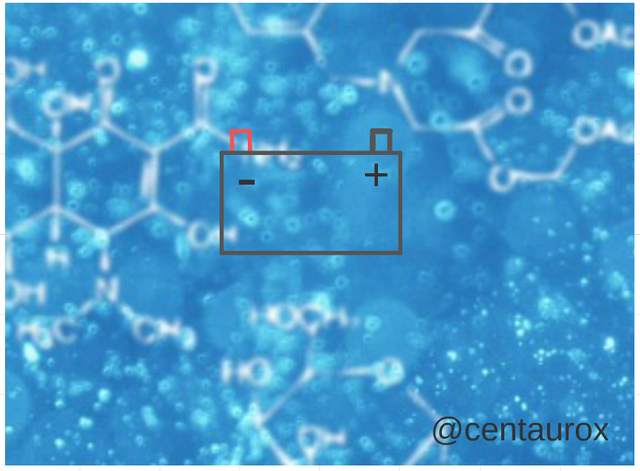
In the technology, in 1859, the physicist and French inventor Gaston Planté developed the battery of lead - acid, considered the first electrical rechargeable battery. Dioxide of lead (PbO2) Cathode, The battery is formed by a deposit of sulphuric acid and inside him a set of badges of lead, parallel between yes and arranged alternadamente as for his polarity (print () and denial (-). To avoid the curve of the positive badges, one arranges a negative additional badge, so that beech a negative exterior badge, Acid is always a solution of Sulphuric Acid and (distilled) water, with a specific thickness that might be established inside a status of 1.200 gr/cc to 1.400 gr/cc, these values are defined in accordance with the capacity of the battery, use, temperature, diet of discharge, design, materials in the manufacture of the battery between other conditions.
The Bacterium.(Image edited for @centaurox @centaurox)
Production of acrylic acid the éster, by means of a combination of the technologies of Lurgi and Nippon Kayaku, Air Liquide Engineering * Construction, offers the possibility of producing acid acrylic grade éster (EAA), used often in adhesives, paintings and revetments as well as in the production of nappies. The process offers high yields of acrylic acid and a real-time of long functioning, the valuations of consumption of raw material and of energy are low, and the impact on the environment is minimal.

Image edited for @centeurox
Applications of the acid benzoico in the science, medicine and cleanliness. The principal use of acid benzoico is like that of antimicrobial and antifungal agent, although his most common use is to turn the form of his salt (benzoato of sodium), for this intention and with safety restrictions of the benzoato of sodium, the components are connected by the ring of benzene of carbon, oxygen and hydrogen, which consists of an aromatic acid.
we realized that the acids and the bases are in our life daily and it takes many applications an as as also his use in medicines and cosmetics, which talks each other after announces in an educational way and applications scientist, who enriches the knowledge.
Bibliographical Reference.
Garber, C.C. and Tortoiseshell, R.N. (1992). Laboratory statistics. In L.A. Kaplan and A.J. Pesce (Eds.). Clinical chemistry.
Theory, analysis and interrelation. Buenos Aires: Medical Pan-American publishing house, S.A.
Jandel Scientific. ' Sigma Plot 8.0 for Windows ™ '. (Jandel Scientific, Ed.). Jandel Scientific: Cut Madeira, 2002.
You Martínez-salt, J. (1982). Elements of mathematics. Valladolid: José Martínez Salas.
Ravi Kumar, M.N.V. (2000). To review of chitin and chitosan applications. Reactivate * Functional Polymers 46, 1-27.



.jpg)
