Why haven't we cured cancer yet? A report from the forefront of cancer research.
Figure 2: Vaccine-Based Immunotherapy from Novel Nanoparticle Systems.
According to the WHO, cancer is one of the leading causes of death worldwide, accounting for 8.8 million deaths in 2015. Moreover, the number of new cases is expected to dramatically rise by about 70% over the next 2 decades [1].
Even though mankind has fantastically advanced technologies in healthcare and various other fields (e.g. this very decentralized website), how is it possible that we are still struggling to find a cure for a disease like cancer?
The answer lies – like with so many things – in the origin of things.
This article is aiming to give non-scientists an insight into the current frontiers of cancer research. As for me, I am currently working in the intersection of molecular biology and bioinformatics in one of Europe’s leading research institutions. Our aim is to identify novel, prognostic biomarkers by which tumors can be classified for better subsequent treatment. Confused about those terms? No worries, by the end of this short article you will be able to use them yourself.
But first, what is cancer really?
Cancer is when abnormal cells divide in an uncontrolled way. Some cancers may eventually spread into other tissues. [2]
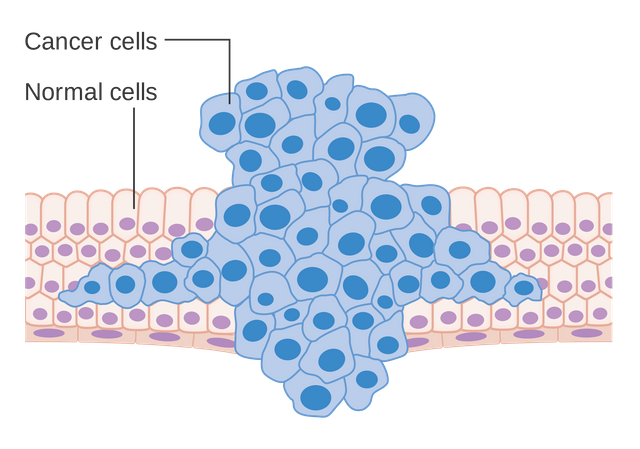
Figure 1: Formation of a tumor forcing its way into the adjacent tissues.
A tumor originates, when one or more cells mutate and, thus, start to behave differently than they are supposed to. Mutations are random processes where genes, the cells blueprint, are altered. Analogously, changing the blueprint of a house under construction leaves the workers with wrong instructions and the house may end up different then it was intended to. Moreover, some of those introduced changes may have more severe implications, especially if they target important structural and functional hubs like maybe the stairs that connect two floors. If a cell now loses an important function or it acquires a totally new function, then this cell is called transformed and prone to become a cancer cell. Fortunately, one mutation alone is most definitely not enough for a cell to form a tumor. In fact, the formation of cancer is a step-wise process during which a cell accumulates many different mutations over time before it becomes malignant. This is also the reasons why elderly people are more likely to get cancer than teenagers. The cells just had more time to mutate. And this is the first key point to take away: cancers are not just cells with one abnormality, they feature many different mutations and abberant functions. Moreover, there are many different reasons why mutations can occur: exposure to chemicals, dietary habits, UV radiation to name a few. Unfortunately, to what exact mutation these risk factors lead is unpredictable.
In summary, cancer is a multifactorial disease that features numerous mutations in unpredictable locations, which result in cancer cells being unique. These unique cancer cells then transform adjacent cells to behave malignantly and recruit them for growth and invasion of other tissues. This differential origination leads to tumor heterogeneity. Importantly, tumors do not only differ between patients, but also within the same patient and, surprisingly, even within the same tumor. Why? Because cancer cells in different locations may acquire mutations that differ from the other tumor. Their blueprints change and with them their malfunctions. There are numerous ways how cells can be cancerous, but they all share certain functional hallmarks like rapid cell division, invasive migration and evasion of the immune system. And this is exactly where all cancer treatments aim at. In order to treat cancer, we need to find a way to overcome this massive heterogeneity and find something that enables us to target all different cancer cells. Furthermore, we not only need to find a pan-cancer marker (a marker shared among all cancer cells in all patients) but also make sure all normal, healthy cells do not die.
This tradeoff between generalization and selectivity makes treating cancer very difficult.
Until very recently, chemotherapies have been the only option to treat cancer. The former are essentially drugs that target one of the cancer hallmarks I have pointed out: rapid cell division. Unfortunately, there are other healthy cells that divide rather fast, for example the hair follicles and the immune cells. For this reason, patients undergoing chemotherapy show strong side-effects like hair loss and a breakdown of the immune system. Chemotherapies generalize well across cancer cells, but are not selective enough across all cells.
But how can we overcome this selectivity issue?
The answer is: so far, scientists are really struggling. In my and many other scientist’s research, we aim to target this exact problem: we try to find novel markers (proteins, genes, metabolites etc.), biomarkers, that are only present in cancer cells and not in healthy cells. This way we can literally mark those cells and analyze them directly. Despite this sounding like a good idea, we have found not a single biomarker that is shared across all cancers. The solution: personalized treatments. Instead of trying to find a treatment that works for all cancer types and patients we limit our focus on finding treatments that work for a specific patient. This idea gave rise to a new approach called: immune therapy.
In general, the immune system is able to identify and kill cancer cells. This happens every day in all of us. The issue arises when the cancers mask themselves as healthy cells and evade the control of the immune system. Immune cells recognize cancerous cells by checking their surface for specific biomarkers. Eventually, some cancer cells mutate and lose those cancer biomarkers or gain the ones of healthy cells and continue to grow. Immune therapy tackles this issue by introducing new biomarkers on the cancer cells, so the immune cells are again able to recognize and kill cancer cells.
Immune therapies work in three steps.
- Find selective biomarkers that distinguish cancer cells from healthy cells (patient-specific)
- Use those biomarkers to literally mark cancer cells (antibodies against the markers)
- Program the patient’s immune system to recognizes the introduced biomarkers and kill the cells.
In theory, immune therapies could be the way to defeat cancer once and for all. However, finding new biomarkers for each patient individually is neither trivial nor cheap. Furthermore, the reprogramming process of the immune system is a fairly new strategy that has shown to be highly complex and difficult to perform.
In sum, cancer is a complex, multifactorial disease which results in a massive heterogeneity of tumors within and across patients. In recent years, more and more researchers have come to the conclusion, that there cannot be a single, magical drug that heals cancer. On the contrary, personalized treatments, especially: immune therapies, aim to find patient-specific biomarkers that support the native immune system to kill cancer. These days, the war on cancer is rapidly changing gears away from simple and generalizing towards more complex and selective treatments.
[1] http://www.who.int/mediacentre/factsheets/fs297/en/
[2] http://www.cancerresearchuk.org/about-cancer/what-is-cancer/how-cancer
Resteemed by @resteembot! Good Luck!
The resteem was payed by @greetbot
Curious?
The @resteembot's introduction post
Get more from @resteembot with the #resteembotsentme initiative
Check out the great posts I already resteemed.