Pollution Free Electricity Generator - Alkali Fuel Cell
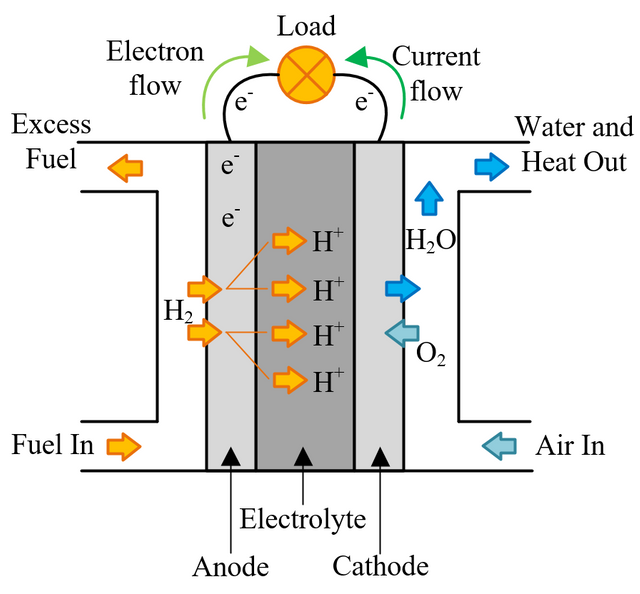
In a conventional power plant, fuel and air enter the power plant and products of combustion leave the unit. There is also a transfer of heat to the cooling water and work is done in the form of electrical energy. The fuel cell accomplishes this objective by converting the chemical energy of a fuel directly to electricity. A schematic arrangement of a fuel cell of ion-exchange membrane type. In this fuel cell, Hydrogen (H) and Oxygen (O2) react to form Water(H2O). The flow of electrons in the external circuit is from anode and cathode.
Fuel Cell Work
In this cell hydrogen and oxygen gases flow from carbon electrodes with foramen to (Concentrate Potassium Hydroxide) KOH solution as bubbles. Hydrogen gas is sent to the anode chamber, where it is oxidized and the oxygen gas is sent to the cathode chamber, where it is a reduction. The cell's potential at the equilibration of the cell is 1.229 Volts.
Each electrode is made of carbon containing foramen, which contains Pt, Ag or Cao in small amounts, which acts as a catalyst. until reactant is sent, this cell works continuously.
When the anode and cathode are connected with an external load, the reaction begins in the cell, in this reaction, the Combustion of H2 and O2 gases creates H2O.
The reactions are as follows:
Anode Reaction 2[H2(s) + 2OH-(aq) → 2H2O(t) + 2e-]
Cathode Reaction O2(g) + 2H2O(l) + 4e- → 4OH(aq)
Overall Cell Reaction 2H2(g) + O2(g) → 2H2O(l)
At present, the fuel used in fuel cells is either hydrogen or a mixture of gaseous hydrocarbons and hydrogen. The oxidizer is usually oxygen. However, current development is directed toward the production of fuel cells that use hydrocarbon fuels and air.
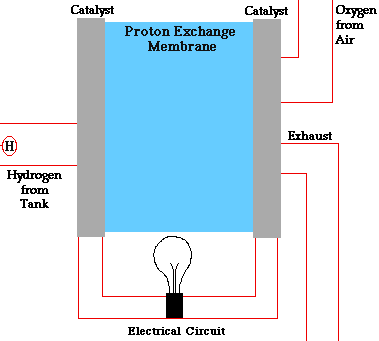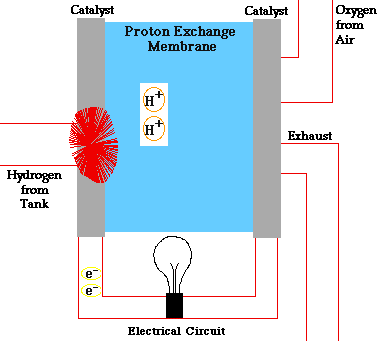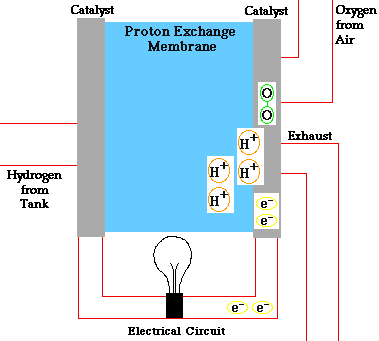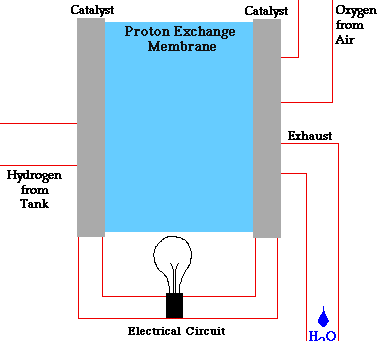
Image Source
THermodynamics plays a vital role in the analysis, development and design of all power-producing system, including I.C. engines and gas turbines as well as the power-absorbing system like refrigerators and air conditioners. Considerations such as the increase in efficiency, improve design, optimum operating conditions, environmental pollution and alternative methods of power generation, involve, among other factors, the careful application of the fundamentals of thermodynamics.
The Hydrogen-Oxygen Fuel Cell was used to generate electric power in the Apollo space program. The water obtained from this was used by astronauts to drink. The weight of enough fuel for eleven days was about 200 kilograms, which was very less than the other tips to obtain electricity.
Some special advantage of fuel cell
- The energy of the fuel cell can be converted into electrical energy directly, its efficiency is higher than other methods of generating electricity.
- The efficiency of the fuel cell is up to 60-70% per cent, but in other methods(Thermal Power), efficiency is available up to 4% per cent. So it is suitable for spacecraft.
- Fuel cell pollution is free.