Saponification 1/2
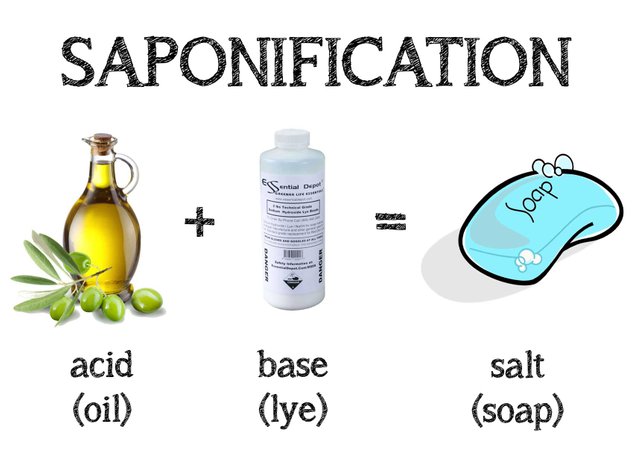
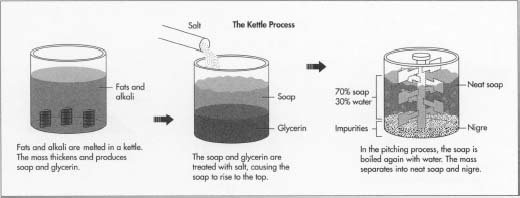
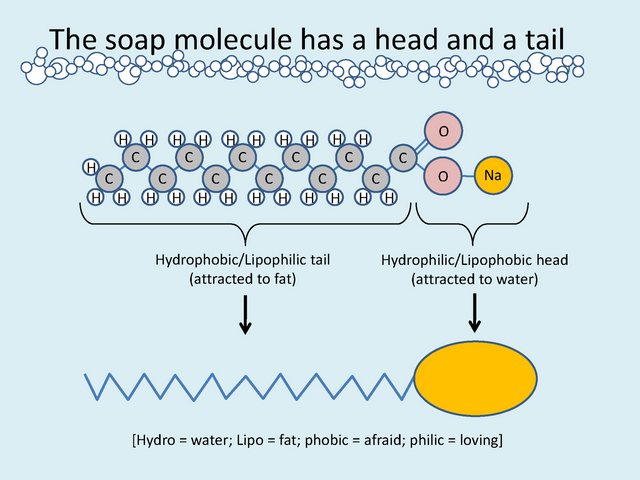
The chemical reaction which is used to produce or form soap is know conveniently as the saponification reaction in which case the esters or fat (glyceryl ester) are used in a solution where lye is combined with water and mixed with an alkali and ester similar to sodium hydroxide or potassium hydroxide to produce a free alcohol (glycerin) and a carboxylate salt, to summarize alkaline hydrolysis of a oil or fat to produce soap. Ancient men have produce soaps from cooking animal fat when the fat or oil dripped into the fire it produced a chemical reaction when the ester combined and reacted with the wood wash to form the soap after a period of time.
Now lets start a fight club...
Don't drop the soap :)
formula best , nice

Congratulations @doctortesla! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPThank you so much for sharing knowledge.
I've got some knowledge!
Please vote me
Please follow me