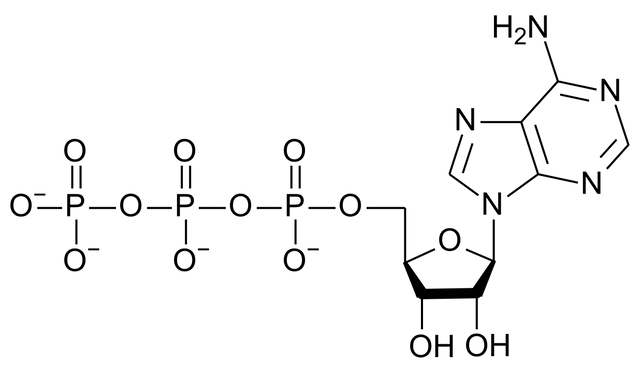
Why gels retain water, electrostatic attraction and charge polarization between water and adsorbing material
Update: another possibility is that the "surface phase" is paramagnetic, and weakly attracted to the nucleating material. And, draws the protonated hydronium in the water near it with it, electrostatically (and as this layer can extend some bit outwards, the bulk of water there behaves as a positively charged mass, and is retained by the nucleating material. ) This relies on that the (H3O2-)n phase "sticks" to the material because it acquires an eletromagnetic field that is attracted to the material, and the protons it ejected that diffuse some way out into the water nearby are in turn attracted.
One model to explain how gels keep their water, is that they are charge polarized. The water could be one pole and the material it binds to the other. This could result from that protons from the water enter the material the water binds to, making it slightly net positive, and the water becomes slightly net negative and alkaline. The cytosol in cells is slightly alkaline, with a pH of 7.4, and often claimed to be negatively charged. The effect of water pressing against the material is to form a “dense solid phase” of water, that is itself charge polarized, and that has an inherent effect to “pump” protons into the material. This supports that the material itself might get protonated.
Protonated peptide backbone
In peptides, these protons could for example be stored in the resonance state of the protonated carbonyl group, and the amine group, that take turns storing a positive charge as the double bond alternates between the two.
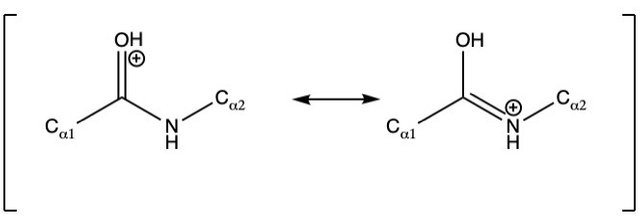
Peptide bonds have this resonance behaviour (image below, left), and that protonation would be favoured on the carbonyl group is similar to how protonation of a carboxyl group works (image below, right. )
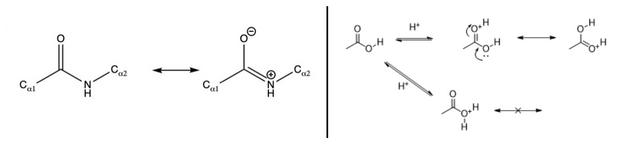
There are plenty of peptide bond sites in protein because that is pretty much the definition of a protein, all amino acids in a protein are attached by peptide bonds, forming the "peptide backbone".
The role of ATP
ATP is a base with a negative charge of 4 at neutral pH. As proteins in cells are pumped full of ATP (the ATP binding to ATP binding site, and at this site the triphosphate tail of ATP binds to the p-loop motif), ATP is also able to take up protons. Keep in mind that the inherent effect of the interfacial water covering the proteins, is to pump protons into the protein (to equalize charge distribution locally. )