How Cells Use Logic To Do The Impossible
Curtain open
Pick up any introductory biology textbook, and out of the handful or so defining properties of life that they list with little variation, there is one that seems to stand out particularly well: life's ability to create and maintain order. [1]

Life is highly ordered.
Source: Wikimedia Commons contributors, modified
The reason this quality stands out more than the rest, is because life's propensity to form order seemingly defies one of the cardinal laws of nature: the second law of thermodynamics, which states that the entropy of a system never decreases.
A more vernacular — and more terrifying — way some choose to state this law, is to say that disorder always increases, and the universe tends toward randomness, or "corruption". Check out this TED talk if you've been wanting to watch a horror flick but can't find any that has good IMDb ratings. (I highly recommend watching this talk. By the end of it you'll have become scientifically convinced that absolutely everything you do makes the universe worse off!)
Viewed in these terms, life is fundamentally antithetical and antagonistic to reality. In this post I'll zoom in on just one of the many ways in which life battles pernicious disorder, and is, however temporarily, able to gain the upper hand. Specifically, I'll talk about how cells use logic to make otherwise impossible chemical reactions move forward, in what is more formally called "reaction coupling", but which I just like to call "cells that know their Aristotle"!
A detour
But first, we need to know a bit about Gibbs free energy, to understand the kind of impossibility cells literally have to surmount.
In order for cells to provide us with the energy that will lead to the orderliness that we perceive every time we stand in front of the mirror (actually, I think one of my nostrils is bigger than the other; but I digress), each one of these cells needs to perform "millions of reactions every second" [1].
Some of these reactions are spontaneous, meaning they will happen on their own, without external energy input. This is expressed in Gibbs graphic formulae like so:
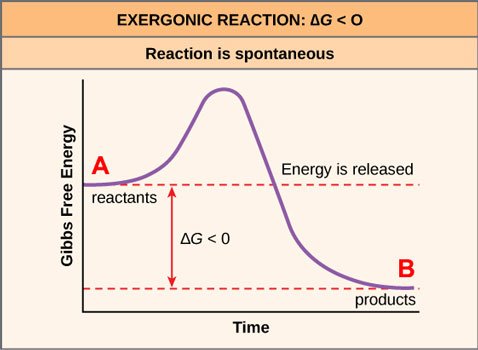
Source: OpenStax, Concepts of Biology, modified
I've labeled the product as B and the reactant as A. B is lower than A, which in Gibbs lingo means that B is more energetically favorable than A. (Here's a mnemonic device: it's easier to go downhill than uphill.) The reaction A to B, written as A→B, will therefore move forward spontaneously.
If you remember the second law of thermodynamics, you already know what this means: A→B is a move from order to disorder.
Yikes!
If you have the kind of inquisitive brain that I do, this still doesn't make sense to you: why is a move from order to disorder easier?

A highly ordered clothing store is one rife for disorder!
Source: Pixabay
To explain this using an everyday example, think of a clothing store that does not have employees to refold and replace the clothes that customers try on. Will that clothing store tend toward disorder, or toward order? Common sense says it will tend toward disorder. But why?
The reason is because there is only one way for order to be achieved — the one that the designers of the store have deemed ideal — and a great number of ways in which it can become disordered (especially if the customers bring their kids along). So, by the mere laws of probability, the store will become disordered — unless employees are hired to actively work to maintain order. Here's a way to graphically represent what the employees do:
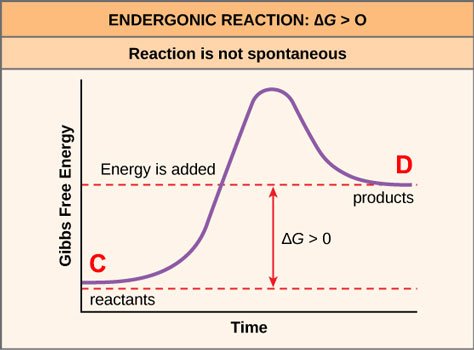
Source: OpenStax, Concepts of Biology, modified
So the reaction from disorder to order (C→D) in this case is unfavorable, and requires employees expending energy in order to drive it forward, and sometimes these employees must surmount significant psychological barriers in order to force themselves to keep up their work and/or not to lash out at the sheer thoughtlessness of some customers!
Again, I find it very useful to refer back to our second law of thermodynamics: after all, all these parochial examples are just specific instances of global laws.
So if we think of it in terms of the second law of thermodynamics, sometimes order and disorder are easy to grasp intuitively. For example, if you know that a catabolic process takes the food you consume and breaks it down into smaller particles, and an anabolic process takes those small particles and builds them back up into proteins your body can use, which process do you think will require more energy? In other words, which process represents the Gibbs graph that moves from order to disorder, and which process represents the Gibbs graph that moves from disorder to order? Hopefully your answer will match this image:
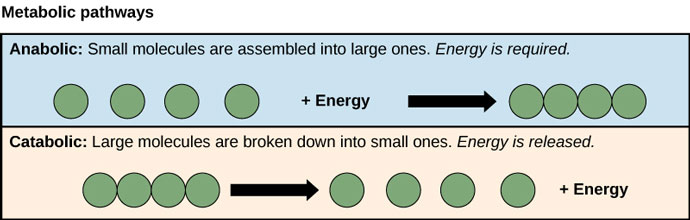
Source: OpenStax, Concepts of Biology
Even in catabolic processes, however, some work is often required. This is represented in the Gibbs graph as an activation barrier: it's the little "hill" or "bump" you see in the middle (we'll revisit it later). The reactant is quite happy being where it is, in its little organized state, but a spark is all it takes to move it forward (over the activation barrier). A wood won't spontaneously catch on fire, but once a spark has lit it, beware forest!
I like to liken the activation barrier to what in human psychology they call status quo bias or resistance to change. We always favor our default position, and have to be pushed over an "activation barrier" (in the form of a well-presented argument, for instance) to adopt a new one. It appears we are like cells in this too. Metaphorically speaking.
So what do cells do about activation barriers?
Enzymes

You're not having a déjà vu: this is the same graph as the first one.
Source: OpenStax, Concepts of Biology, modified
Going back to our spontaneous A→B reaction, what's interesting to note here, is that the graph tells us the reaction will take place, because the product (B) is in a lower (more "favorable") energy state than the reactant (A). However, it doesn't tell us how long the reaction will take. For all we know, it might take centuries for all the reactants to become products.
Now I don't know about you, but I don't have hundreds of years to wait, and neither do my cells, which, as mentioned above, have to perform millions of these reactions per second in order to keep me alive.
In order to make these reactions happen faster (in order to make the graph less of a bumpy ride and more of a slide), cells recruit enzymes, which are proteins whose job is to speed up, or catalyze, reactions. "Enzymes are among the most effective catalysts known, speeding up reactions by a factor of as much as 10^14" [1] Yes, that's a 10 followed by 14 zeroes!
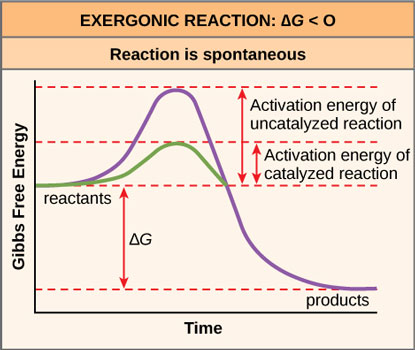
Source: OpenStax, Concepts of Biology
When cells do logic
But what happens when the product is higher than the reactant? Cradle as it may, the enzyme cannot turn an unfavorable reaction into a favorable one: all it can do is lower the activation barrier. What does a cell do then?
C to D (C→D), as you remember, is an unfavorable reaction. But A to B (A→B) is a favorable reaction. Let's say it's a very favorable one. What if the enzyme handles both these reactions, so that their product is a favorable one? In other words, what if there was an enzyme that took A+C and gave us B+D (A+C→B+D)? Now we've made an unfavorable reaction into a favorable one! It's not much unlike a businessman using his flourishing business to keep his failing (but promising) one afloat.
The mathematically trained cell often uses ATP (adenosine triphosphate) to make this happen. This is the reason ATP is considered the energy currency of the cell [11]. Whenever a cell wants to make something energetically unfavorable happen, it turns to ATP. The enzyme takes the ATP, removes a phosphate, turning it into an ADP (adenosine diphosphate).
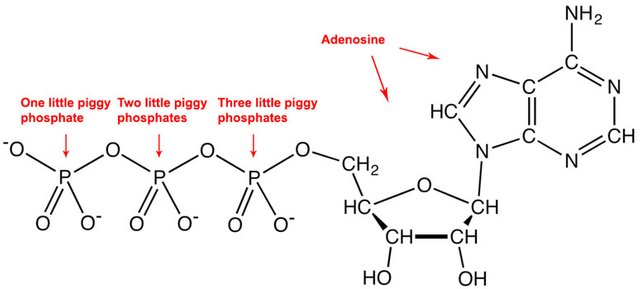
I made the counting from left to right, but if you're naming them for a test, then you had better go the Japanese way!
Source: Wikimedia Commons contributors, modified
If you've been paying attention, you don't need to know the details to understand why breaking apart a molecule ("creating disorder") releases energy. The ATP however is particularly reactive — chafing at the bit, so to speak, with those negatively charged (-) phosphates right next to each other, pushing each other away — which is why the cell uses ATP to get energy when it needs it, coupling it to unfavorable reactions in order to drive them forward. More than a hundred of these ATPs are in a typical cell at any moment, ready to "fire away", and in many cells this amount is used up and replaced every couple minutes [2].
Logic 2.0
There is something else that determines energetic favorability: it's not just absolute like the simplified model shown in the Gibbs graphs. The concentrations matter. If we've got a lot of Bs and very few As, the solutions will reach an equilibrium. Some of the Bs might even start going back to As, even if the reaction, absolutely speaking, favors the reverse.
To make this clearer, let's go back to the clothing store example for a minute. If the clothing store is ordered (A), then it is highly likely that any change will make it more disordered (B). If, however, the clothing store is highly disordered (many Bs), then the reaction could run backwards: a customer, no matter how thoughtless, might make a change that actually makes the store more ordered (B→A).

A highly disordered clothing store is itching for some order!
Source: Flickr
So what does the cell do in that case?
It takes a formal logic class, of course.
And after that, it knows what to do. Since the problem is too many Bs, turning Bs to something else, like Cs, will make As start turning to Bs again. It's a chain reaction! Or, rather, a chain suction! It's like sticking a straw at the bottom of the drinking glass instead of the top, and by sucking the lower liquids you're helping the top ones come down as well.
And there's no limit to the number of reactions I can do this to. It can be A→B→C→D→E→F and so on, I can pull forward a great number of reactions by just manipulating the last one.
When the reactions are linked together in long series like this, they are called pathways. They can get quite involved. More involved than the New York City Subway.
Yeah, I guess that's a good place to stop then!
Gladly we don't have subways where I'm from. We do have Subways though. Yummy!
Source: Wikimedia Commons contributors
Curtain close
Biology had invented mathematics and logic long before us. Well, strictly speaking, their workings can be described and understood in mathematical and logical language.
And that's how I like it. I like being able to understand the general, and derive the specific. There's just too many specifics to remember! But if you're able to really grasp the general — like the second law of thermodynamics, for instance — you can get a handle on any specifics life throws at you. Hopefully I've been successful in not weighing you down with the biochemical details, but helping you appreciate the principle of it, the mathematical logic of the thing.
And it can teach us a lot, this logic. Cells, you see, are very effective. For example, cells harvest nearly 50% of the energy that could potentially be derived from the oxidation of glucose and fatty acids; in comparison, a typical human-made car engine can turn no more than 20% of its fuel's potential energy into useful work [2]. I know, pretty lame! As aggregations of cells, we ought to do better than that!
References
Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. Catalysis and the Use of Energy by Cells. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26838/
Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. How Cells Obtain Energy from Food. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26882/
Wikipedia contributors, "Second law of thermodynamics," Wikipedia, The Free Encyclopedia, https://en.wikipedia.org/w/index.php?title=Second_law_of_thermodynamics&oldid=812704510 (accessed November 29, 2017).
Wikipedia contributors, "Entropy," Wikipedia, The Free Encyclopedia, https://en.wikipedia.org/w/index.php?title=Entropy&oldid=812587662 (accessed November 29, 2017).
Atkins, Peter. (2015, July 1). The second law, why worse can be better | Peter Atkins | TEDxOxBridge
OpenStax, Potential, Kinetic, Free, and Activation Energy. OpenStax CNX. Sep 30, 2015 http://cnx.org/contents/16b284a1-de63-4e08-910a-8baf1b94fc1e@7.
OpenStax, Energy and Metabolism. OpenStax CNX. Jun 1, 2013 http://cnx.org/contents/ed5df737-77ff-452c-b91e-a2c2dad59606@8.
Wikipedia contributors, "Status quo bias," Wikipedia, The Free Encyclopedia, https://en.wikipedia.org/w/index.php?title=Status_quo_bias&oldid=810037779 (accessed November 30, 2017).
Furnham, Adrian. Resistance to Change. Psychology Today. https://www.psychologytoday.com/blog/sideways-view/201610/resistance-change
Wikipedia contributors, "Metabolic pathway," Wikipedia, The Free Encyclopedia, https://en.wikipedia.org/w/index.php?title=Metabolic_pathway&oldid=811366067 (accessed November 30, 2017).
Wikipedia contributors, "Adenosine triphosphate," Wikipedia, The Free Encyclopedia, https://en.wikipedia.org/w/index.php?title=Adenosine_triphosphate&oldid=811954010 (accessed November 30, 2017).
Earlier Introduction to Biology episodes:
3 : Armchair Science — The Discovery of Proteins' Secondary Structure
2 : How Cell Membranes Form Spontaneously
1 : Eduard Buchner: The Man Who Killed Vitalism
steemSTEM is the go-to place for science on Steemit. Check it out at @steemstem or browse the #steemSTEM tag or chat live at steemit.chat
The laws of nature over powers any man made law.
I never thought of the ways cells input energy into reduce entropy as "logic," but now that you've described it I like the metaphor. Thanks for the post.
Thanks for reading )
Communication inside the human body is tremendous. The foods we eat literally speak to our DNA. These elements and minerals have consciousness. Logic results and order and disorder just kind of happen. I guess our whole lives are spent just simply trying to hold it all together I guess. I hope this doesn't sound pessimistic because disorder throughout our physical world is what makes what's inside our own head even better. Our who'e world is inside of our mind. At least it makes me feel calm and I'm a pretty optimistic person who sees the order of things even in disarray. Your post was intriguing. Captured me!
This article reminded me of so many beautiful memories. My whole world changed when I started learning about entropy. What I find beautiful about the cells ability to find order is that the cell represents all of life.
We are made up of cells (organs) that are made up of smaller cells. We ourselves are cells and the oraganisms come together to make organs for the ecosystems that then become the organs to our entire planet which is itself an organism.
I've been reading about the bacteria that live in cloud to write my latest steem article and relishing at lifes ability to persist.
Your post is today's subdaily scientific resteem. Subdaily because I am backpacking in Columbia and wifi is not consistency in my life. I do this to support a growing scientific community of thinkers and doers on steemit. Thanks for inspiring learning and thought!
Thank you for reading and commenting!
In one of my earliest encounters with a nihilist, when I was a teenager, he summed up his argument with one word, "entropy"! It was then that I took an interest in this. It's interesting you find beauty in the same thing he found so dooming.
There is some good stuff here. As a researcher in philosophy, particularly Plato and Aristotle, I find the general thrust of this piece fascinating. I believe Aristotle would too have agreed--though you would have had to go a long way to get him to agree--that cells "use" logic. Though for Aristotle it could only have been the same way an animal "thinks", which was more or less "automated"--with some interesting exceptions. That said, they could not be "choosing" to use logic; even if they were using it. This is more or less Plato's critique of logical reasoning, but that is really another story.
The more interesting line of thought, IMO, is from the Myth of Silenus. Silenus was a buddy of Dionysus, the party god, who was said to have been extremely wise; especially when drunk. When forced to explain the meaning of life, Silenus replied: If human, best never to have been born at all. Second best is to die soon. Uh, yeah.
Sounds like Silenus was already hip to the idea of final chaos. There is a new movement in philosophy called anti-natalism, which is just what it sounds like. Best NOT to have kids, never be born at all. Since life is more pain than pleasure overall, in that sickness and death out weight the rest.
I see interesting links between your ideas and this new movement.
I actually have a book called Better Never to Have Been, which I read years ago. I didn't realize there was an active movement within philosophy, though I do see it in real life very much, people opting not to have children.
Thanks for the interesting comment!
HA! The author of that book is the one being somewhat credited with starting the movement. It is really ancient, obviously, but academics like to give things new names all the time so they can feel relevant. Silly scholars.
I have no intelligent comment to do with respect to your post, but I just wanted to let you know that I have appreciated the reading. Some good old thermodynamics applied to biology :)
You're an established figure here. Established figures don't have to make intelligent comments! Just giving us attention is enough! :P
You do a great job making this complex subject engaging.
But those clothing store photos are giving me PTSD flashbacks to my retail management days!
Oh yes I remember that from one of your posts!
Apologies! :P
"We always favor our default position, and have to be pushed over an "activation barrier" (in the form of a well-presented argument, for instance) to adopt a new one. It appears we are like cells in this too. Metaphorically speaking. "
This is absolutely beautiful. What a great article. Resteemed :)
Oh thanks )
Great insight @alexander.alexis thanks for sharing, I've always thought the concept of entropy was an interesting one and the universe as a whole will always tend to disorder.
Long time no see - glad to find you exploring new ways, to us, for us to combine philosophy and biology. I never was good at school, in biology, arithmetic or anything that require a strict 'follow-the-leader' of logical trains of thought, I mostly did not do well where I had to memorise...but always enjoyed the hiccups in my mental world when I try to open new pathways of intuitive logic - even if they tend to lead me astray.
In other words, I knew, as a schoolboy, that food goes in the one end and something happens in the middle and then out comes that disgusting stuff I know I never ate...so what the hell is it doing coming out of me? Once, I got so stubborn about denying it, that I was constipated for just under one month. When the teachers found out, they brought a bag of oranges and told me I have to keep on eating them until I go. Luckily it happened within three hours, which saved me from having to down another orange, which I was sick of by then
I presume your end of the world is turning cold; it is supposed to be summer here, but our wild and exciting thunder and lightning storms have been cooling us down (it hailed today, which was nice for me, but not for those who have farms).
On the serie of 16 of yours, I am still on #9. The good thing is, at least 1 to 4% of it sticks, so I am slowly growing, thanks to you.
I watched a video of Jonathan Bowden and he talked about Nietche, Value and so on, and it made me think of you.
Rote memorization never sat well with me either! I could only take an interest in something if it seemed of profound importance and if I was allowed to be creative in it. Matters that seemed settled didn't seem to be worth learning. At uni (unlike school) teachers often tell you about fringe areas of their subject where many questions are still unsettled. I loved uni. I wish school would be more like it.
Funny constipation story! I guess the same is repeated in many different forms and areas of human life. For instance, religion does it a lot, trying to deny that humans are such-and-such or could do x and y.
It's cold-ish here. Today it was warm and sunny though. Got myself some vitamin D!
I'm glad that steemit provides an alternative form of learning. So many people have a problem with the traditional classroom setting and they miss out on so much amazing information. It has always been one of my life mission to find and creat alternative forms of scientific learning. Often I've been doing scientific murals and telling people about the structures and things I paint while I work. Now I'm also writing scientific articles for steem! Im curious why oranges were the thing they had you eat. I guess they are watery but maybe the citrus has something to do with digestion.
Each segment is wrapped in a fibrous material. It does help with keeping a tummy regular - but, I tend to believe in magic of suggestion. They had me thinking the oranges will work, so they did.
Mind over matter is quite an exciting subject, for it has no negative aspects and should help us grow stronger and more positive.
Some years ago, I had taken a meditation course in London and it was evening and I had a toothache. I suddenly realised I cannot close my mouth because the gum has swollen to the point it is almost covering the tooth. Dentists are not easy to find at night, so I closed my eyes and imagined millions of tiny workers in white coats rushing to fix my tooth and gum.
Suddenly I realised my mouth is closed. I felt and found my gums had gone back to their regular size and shape and my tooth no longer hurt.
I've never managed to do anything like that again, but, as they say, the exception proves the rule.
As for education, some important experiments took place, before all goverments decided to mess up our kids with the worst systems possible. We threw away such a golden opportunity to improve ourselves and the world. So, good luck to you. Any attempts are bound to make a difference, even if only to a few. That counts.