The Building Blocks of Matter
Atoms are the fundamental building blocks of matter, forming everything we see, touch, and experience around us. But what exactly is an atom? How do atoms interact to create the diversity of materials and elements we encounter in our daily lives? Let's dive into the atomic world to understand these tiny particles that shape the universe.
What Is an Atom?
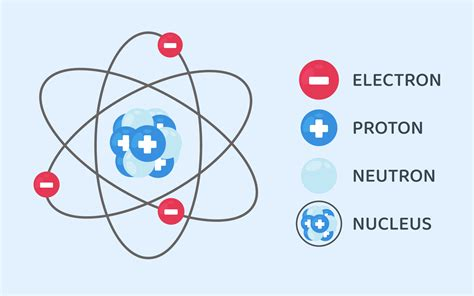
An atom is the smallest unit of ordinary matter that retains the properties of an element. Each atom consists of a central nucleus surrounded by electrons in orbitals. Atoms combine in various ways to form molecules, materials, and all kinds of substances, making them essential to life and everything that exists.Structure of an Atom
Atoms are composed of three main particles:
Protons: Positively charged particles found in the nucleus. They determine the atomic number of an element, which defines its position on the periodic table.
Neutrons: Neutral particles, also found in the nucleus. Together with protons, neutrons contribute to an atom’s atomic mass.
Electrons: Negatively charged particles that orbit the nucleus in energy levels. The arrangement of electrons determines how an atom will interact with others.
These particles work together to create a stable structure, with positively charged protons balanced by negatively charged electrons.
Atomic Theory: A Brief History
The concept of the atom has evolved significantly. Early Greek philosophers like Democritus theorized that matter was made up of indivisible particles, which he called "atomos." But it wasn’t until the 19th century that John Dalton, an English scientist, proposed the modern atomic theory, suggesting that each element consists of identical atoms and that chemical reactions involve the rearrangement of these atoms.Atomic Bonds: How Atoms Come Together
Atoms combine through various types of chemical bonds:
Ionic Bonds: Occur when atoms transfer electrons, resulting in a positive or negative charge, creating ions that attract each other.
Covalent Bonds: Atoms share electrons, forming stable molecules, such as water (H₂O) or oxygen (O₂).
Metallic Bonds: Found in metals, where electrons flow freely among a lattice of metal atoms, creating conductivity and strength.
These bonds enable atoms to form the diverse materials we rely on, from metals to complex organic compounds.
Isotopes and Atomic Variability
Atoms of the same element can vary in the number of neutrons they contain, leading to different isotopes. For example, Carbon-12 and Carbon-14 are both carbon atoms, but Carbon-14 has two extra neutrons. This variability is crucial for applications like radiocarbon dating and medical imaging.The Significance of Atoms in Our Lives
Understanding atoms helps us grasp the underlying mechanisms of chemistry, biology, and physics. From the oxygen we breathe to the technology we use, atomic interactions play a central role. Advances in atomic theory have led to breakthroughs in energy, medicine, and materials science, shaping modern civilization.
Conclusion
Atoms are small yet mighty, forming the foundation of everything in existence. By understanding their structure, behavior, and the way they bond, we gain insights into the natural world and can harness this knowledge to develop new technologies. Next time you look around, remember: every object, from the smallest grain of sand to the largest building, is built on the remarkable principles of atomic science.