Aquapononics tech#4 Water Chemisty part 1
pH in aquaponic systems #1
pH is one of the first things we need to understand. We need to know
how pH affects our system and how our system affects pH. Let me
explain what that means.What does pH stand for? The abbreviation stands for potential
Hydrogen.
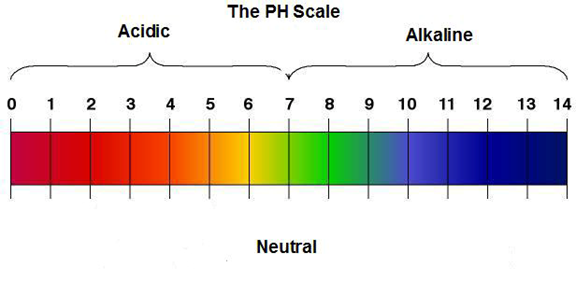
H2O is rarely pure, in most cases it will be either acidic or Alkaline.
Look at the scale diagram. Seven is neutral, Notice that all numbers
below 7 are acidic, and all numbers above 7 are alkaline.
It all works around the Hydrogen ion (H). Some molecules will wind up
splitting or dissociating in any given water solution. This happens when
hydrogen ions (tiny negative charged particles) move around and
exchange with the Oxygen molecules.
Acidic water is said to “donate” hydrogen ions, creating an abundance
of hydrogen ions in the solution.
Alkaline water is said to “accept” hydrogen ions, and shifts in the
opposite direction as the acidic water solution. Alkaline water contains
more hydroxide ions.
The pH scale is done in units of 10
So a pH of 6 is 10 times more acidic than a pH of 7. A pH of 3 is 10,000
times more acidic than pH of 7. And it is the same on the alkaline side of the scale. Each number in the scale represents a x10 increase or decrease in either acidity or alkalinity.
7 is neutral water, but in our aquaponics we will be looking to maintain
a pH of 6.8 or close to it.
Lets dig a little further. Here is a chart from
http://www.sciencebuddies.org/science-fairprojects/
project ideas/Chem_AcidsBasespHScale.shtml
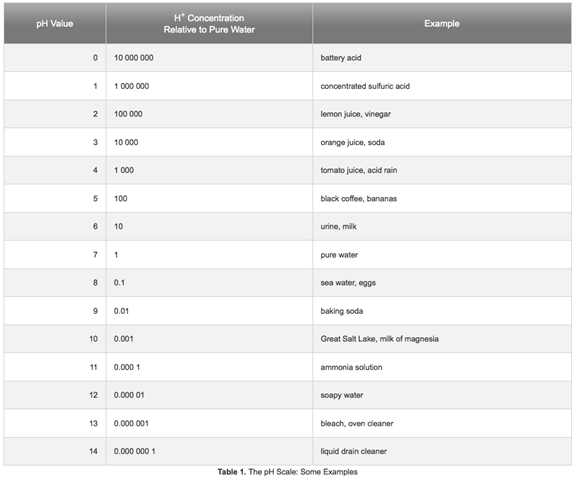
First lets consider the alkaline condition, and look at what we are likely
to encounter at the beginning of our setup. Ammonia is the key
ingredient needed to establish our beneficial bacteria.
Look at where Ammonia falls on the scale. A pH of 11 is highly alkaline.
pH 11 is not conducive to our bacteria growth. Ammonia by itself is
highly alkaline, but since it is part of the whole system it interacts with
other compounds and biological elements, and changes its ionized form
based on the pH of the water solution it is surrounded by.
The presence of un-ionized ammonia, the toxic form, increases as pH
rises and decreases as pH falls which causes ammonia to become more
ionized. Due to temperature and photosynthesis, the concentration of
un-ionized ammonia in fish laden water is lowest just before dawn and
highest late in the afternoon.
This happens due to hydrogen ion exchange.
Ammonia-nitrogen (NH3-N) has a more toxic form at high pH and a less
toxic form at low pH, un-ionized ammonia (NH3) and ionized ammonia
(NH4+), respectively. In addition, ammonia toxicity increases as temperature rises.
For our purposes its important to note that the higher the pH, the more toxic ammonia is in the system.
In the very beginning of the cycling process before the bio-filter is
established the presence of ammonia will tend to convert to the more
toxic (NH4+) unless the pH is monitored and controlled.
In some cases this will drive the pH up in new systems, especially those
with smaller water volumes (under 100 gallons). This happens because
In the very beginning of the cycling process before the bio-filter is
established the presence of ammonia will tend to convert to the more
toxic (NH4+) unless the pH is monitored and controlled. This is due to the lack of our friendly bacteria that use the ammonia as a nutrient source.
I found that small systems would fluctuate wildly in both temp and pH
at various times during the day.
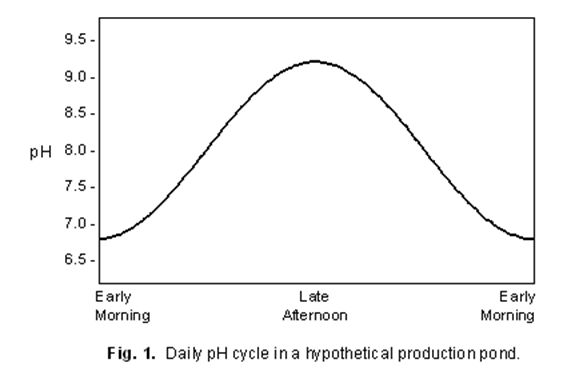
Here is a graph provided by Kentucky State University that shows how
temperature affects respiration of CO2 concentrations, which in turn
affects pH. As you can see CO2 consumption increases as the
temperature increases. As CO2 decreases, it alters the amount of
available hydrogen ions. This will drive the pH up and the ammonia into
a more toxic form (NH4+).
When I built my 55-gallon barrel ponics system I couldn’t figure out for
the life of me why my pH would swing wildly and why I couldn’t get the
ammonia under control. Every water test I did would show off the chart
ammonia levels. I would do a water change and within hours it was
right back. Which led to another lesson in aquaponics pH. This one had
nothing to do with the fish, ammonia, or the size of the system.
All of the courses I had taken said to use either the fired clay balls or
granite rock as the grow bed media. I guess I was suppose to just
intuitively know that much of the granite in the southeast where I’m
located contains veins of high concentration limestone.
As you can see in the next image, I have the Granite rock in the grow beds.
But no amount of acid added to the system would stabilize the pH,
because the granite contained veins of Limestone.
This led to pH increase and increase in the toxicity of the ammonia, but
it also locked up other key nutrients the plants depend on like
phosphorus.
So in this case the grow bed media itself was causing the problem.

You can’t go wrong buying the expanded clay pellets made for
aquaponics. These expanded clay balls are fired and hardened, but most
importantly they are pH neutral.


But what if you can’t afford the expanded clay ball media or if your doing
a large volume media bed and the clay balls are not feasible for you?
How do we know what media will not affect our system in this way?
I devised a simple test that you will want to do when you go to buy your
grow bed media.
Take a mason jar and put about 2 inches of vinegar in it. Before you
purchase any rock for your media, put a few stones in the jar and watch
it for about 30 seconds. If you see bubbles coming off the stones you
know they are interacting with the acid in the vinegar and will influence
the pH in your system. River rock seems to work very well and is usually
pH neutral. But assume nothing. Make sure you test the media before
you buy and install it.
You can use any media you wish, just make sure its pH neutral.
Congratulations, your post has been selected to be included in my weekly Sustainability Curation Digest for the Minnow Support Project.
Host of The Alternative Lifestyle Show on MSP Waves Radio.
Founder of the A Dollar A Day charitable giving project.
Cool.. Thanks.. I just want us (as a society) to get a decentralized sustainable food source in place.. The way I look at it, the more people out there who can grow nutrient rich food for themselves, the more stable our society is as a whole.
Let me know if I can help with anything.