Reactions of oxidation - reduction // your technological application
Greetings for all the people of successful of Steemit, also a big greeting to such big communities as: #utopian-io, #stemng, and especially to @suesa.
according to the classic concept of oxidation, when the iron contacts the oxygen it oxidizes, forming an oxide of iron as the Fe2O3, if instead of oxygen the gas that is wrapped to the iron is the chlorine. This one also reacts with him, forming FeCL3; we can analyze the following thing that both cases the iron has gone on from the elementary state to the iónico, if in the first case it has been said that the iron has oxidized, in the second one it will be necessary to say the same.
it is named an oxidation to the phenomenon for which a chemical species loses electrons and is named a reduction to the phenomenon opposite to the oxidation, that is to say the one that provides profit of electrons, an oxidation is always accompanied of a reduction, since if a substance loses electrons, other one has to gain it. The substance that oxidizes receives the name of differential and the one that diminishes is the oxidizer.
Next we have the following contribution, of several characteristics that they define to the reactions of oxidation - reduction.
1.-The processes that take place in a battery electrochemistry can be analyzed according to two semibatteries.
2.-The reactions of oxidation - reduction can be interpreted as a process of electronic transference.
3.-Atoms and ions compete for the electrons.
4.-The potentials type of reduction of the semibatteries are useful for the prophecy of the chemical reactions.
5.-The equations of oxidation - reduction can balance for the method of the semirreaciones or by means of the employment of numbers of oxidation.
6.-The electrolysis represents a type of useful reaction. In her, a chemical battery binds to work in direction opposite to that of the spontaneous reaction, on having applied a tension major than the produced one by the same battery.
textual Chemical Appointment of the book: experimental essentials. Guide of the teacher for Robert W. Parry; page 188.
The electronic concept of oxidation - reduction does not deny the classic concepts, but they extend them to other substances that it is old theories were neither oxidizers nor differentials. Other substances possess a double character, since according to the substances that they have I faced they can oxidize or diminish, as the following case where the sulfur opposite to a strong differential can limit to ion sulfide: S 2e - = S ²-, whereas opposite to an oxidizer one can transform into an ión sulfite SO3 ²-, of such form what is wanted is to document of educational form, of such a motive in modern theories lose value words as oxidizer or differential, since these concepts happen to be relative and dependent on more one substance. This way the same oxygen, I bring in time excellent oxidizer, they can be a differential opposite to the fluorine.
Conduction electrolytic.
The investigations carried out at the beginning of the XIXth century showed that the watery dissolutions of diverse chemical substances were leading the electrical current in different measurements, to put more skylight these contribute to this content of knowledge, in reference to conductive character of the dissolved substances, it is enough to insert a lamp in a circuit of a simple way, of such a way what is a question of observing his sheen, which will be the indicator of such a way of knowing, what so much is the driver in the solution contained in her.
Electrolysis.
It is the set of chemical reactions that takes place in the electrodes during the electrolytic conduction, it constitutes the electrolysis, taking better understanding as an example apara, we have when the electrolysis of the H2O is carried out, the cationes H move towards the negative electrode (cathode) and the anions OH - towards the electrodes positive (anode).
The reactions that take place in the electrodes are:
ANODE.
4OH-(aq)+2e- = O2(g)+h2O(1) Oxidation.
CATHODE.
2H(aq)+2e- = H2(g) Reduction.
The chemical global reaction, which takes place in the electronic cell is obtained adding the reactions of the anode and of the cathode, so that it gets lost and the same number of electrons is gained.
2H2O(1)= O2(g)+2H2(g) The chemical global reaction.
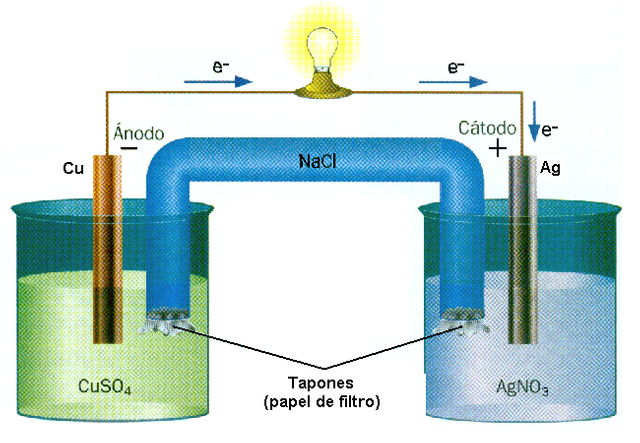
The battery Cu-Ag, an example of a reaction rédox, source of image of mastery of wikimedia commons, Author Muro de Aguas
Contribution of Michael Faraday.
As result of his investigations, Faraday enunciated two following laws, which govern the electrolysis:
1.-The quantity of an element that it settles or liberates in an electrode is proportional to the quantity of electricity that circulates along the dissolution.
2.-The mass of different elements deposited or liberated by the same quantity of electricity are proportional to his equivalent chemists. The quantity of electricity that liberates 1 equivalent gram of any element is 96.496 culombios and it receives the name of 1 faraday.
Force of the oxidizers and of the differentials.
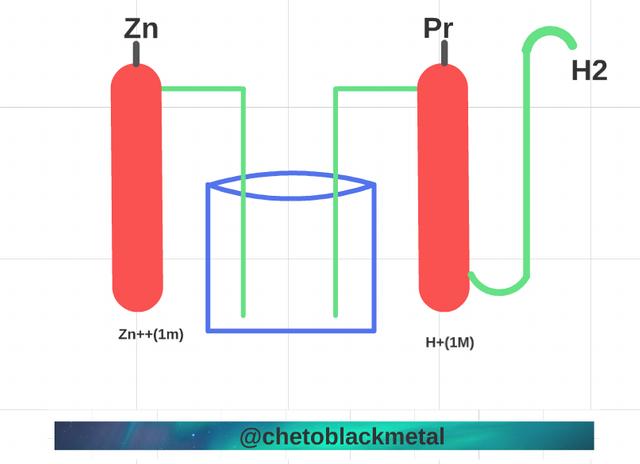
Force of the oxidizers and of the differentials. (Image prepared for @chetoblackmetal)
That's why there are named reactions of precipitation those that takes place between species iónicas in dissolution to give a slightly healthy solid compound and of major thickness than the dissolution.
The Technology and Reactions of oxidation - reduction.
East a very crucial point of science, thanks to his contribution and studies, as the case of the reactions of oxidation - reduction, has great application at technological level, that helped to the making of many hardware, materials, teams that we use in our daily life as they are the following ones:
Also we have applying the electrolysis there was made electrical oven, which is used to make aluminum, magnesium and sodium. Which works of the following way, a load of metallic sales warms up until it is founded and is ionized; in such a way that the metal settles electrolíticamente, this method is applied by the sense to refine and to have the most ideal of purity of the metals as: the lead, the tin, the copper, the gold and the silver.

iss space station,Source of image of pixabay
| Thomas Grubb and Leonard Niedrach | Battery of combustible employee in the missions Gemini |
|---|---|
 Source of image Source of image | 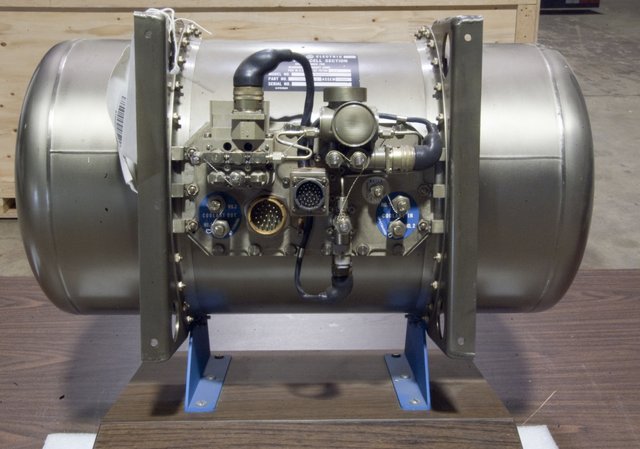 Source of image Source of image |
At present one is employed at batteries of ions of lithium, which support it discharges temperature in the space, also he would say that to support great radiation proveniente of the Sun, in such a way that they are rechargeable with the use of solar cells. What is wanted is to develop source energy you recover free of pollutant and to make use of the source natives, already this this consideration I feel seizure, because it is work and investigating on the application of reactions electroquímicas ecological, with the use solar cell, trying not to damage the environment.

Bibliographical consulted Source.
ou and the chemistry - Page 705 for Andoni Garritz Ruiz - 2001.
Chemistry: experimental essentials. Guide of the teacher - Page 188 for Robert W. Parry - 1974.
Physics and Chemistry 4 THAT - Page 224 for Dulce Maria Andrés Cabrerizo, Juan Luis Antón Bozal, Javier Quarter Pérez - 2008.
General chemistry. Introduction to the Theoretical Chemistry - Page 535 for Cristóbal Valenzuela Calahorro - 1995.

Congratulations @chetoblackmetal! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
Click on the badge to view your Board of Honor.
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard:
I will be featuring it in my weekly #technology and #science curation post for the @minnowsupport project. You will also have the chance to win STEEM right from my own wallet!
Wish not to be featured in the curation post this Friday? Please let me know. In the meantime, keep up the hard work!
If you have a free witness vote and like what I am doing for the Steem blockchain it would be an honor to have your vote for my witness server. Either click this SteemConnect link or head over to steemit.com/~witnesses and enter my username it the box at the bottom.
I wish you all the best here on the Steem blockchain.